Buscar
Results for ''s'
Clear-
Department Spotlight Clinical Research
May 20 is National Clinical Trials Day. Celebrate with us by recognizing the Clinical Research team at Renown Health! The root of every medication, treatment and procedure in healthcare can be traced back to research. From the beginning of the history of medicine, research has always played a crucial role in improving the lives of patients around the world, leaving a permanent mark on how we expand our medical capabilities to this day. Renown Health’s Clinical Research team, in partnership with the University of Nevada, Reno School of Medicine (UNR Med), are leading that effort in our very own community. As our in-house leaders of clinical trials, this team is dedicated to advancing the science of medicine to help further our bottom line of making a genuine difference in the health and well-being of the patients they serve. Trial by (Medical) Jury Every day looks different for the Clinical Research team, especially when it comes to interacting with patients, providers and “sponsors,” which are the organizations providing the treatment for the study. One fact always remains true: communication and collaboration are key, especially among the team who act as the face of this process. Meet Lisa English (pictured above on the far right in a blue shirt), a Lead Clinical Research Coordinator at Renown who serves as the study "project manager." One aspect of Lisa’s day-to-day is seeing patients through their clinical trials from start to finish. It all begins with the setup. “Before we can launch a study, there is a lot of back-and-forth dialogue between everyone involved to ensure the best fit,” said Lisa. “Sponsors will come to us with novel treatments, such as medications or devices, and the inclusion criteria that patients need to meet in order to qualify for the study. We then immediately jump into working with the providers, looking closely at the science and comparing the treatments to what is on the market already.” From there, Lisa coordinates conversations between the providers, sponsors and study teams to gauge everyone’s capacity based on the length of the study, ensuring everyone involved has the time to dedicate to the process. Next, the providers identify patients that meet the criteria for the study, and together, the team decides where the patient visits will happen and discusses any potential barriers that may affect patient retention. The budget is clearly defined at this stage, set up to make sure no patient is ever billed for medical costs incurred as a result of the study. Often, research participants are seen within the specialty clinics throughout the health system, while other times the Clinical Research Coordinators see patients at the recently centralized Clinical Research Office at Renown Regional Medical Center. This location provides an essential public-facing space for the community to learn more about clinical trials and demonstrates the breadth of resources available at Renown to sponsors to strengthen external partnerships and research funding opportunities. Once the study officially begins, team members like Lisa will set patients up for a “screening/qualifying visit.” During this appointment, she makes sure patients get scheduled for their lab work, imaging scans and anything else the provider may need to make an executive decision on whether or not the patient is a good candidate for the study. “I build it all in Epic,” said Lisa. “The study information, directions, requirements and next steps are all loaded in Epic for easy tracking. We are also required to input any notes in the sponsor’s electronic data capture website. All the information I track is inputted without protected health information (PHI), so every patient is completely anonymous.” After the patient officially qualifies, the study goes full steam ahead. Team members like Lisa and the providers receive continual updates from sponsors on the status of the study. “Throughout the entire process, I make sure patients get scheduled for everything that meets the requirements for the study,” said Lisa. “I meet with patients one-on-one to discuss their needs and concerns and ask questions about the study, organize their appointments and charts and deal with any issues or pivots that may arise. It’s very important that every patient fully understands what is going to happen with their care.” The Clinical Research department strives to serve as a care partner to patients, providers and clinics they work with. The majority of our Clinical Research Coordinators are trained phlebotomists and medical assistants, performing their own assessments such as lab draws and electrocardiograms (ECGs) to streamline the research visit process and reduce resource constraints on the clinics and health system. Lisa typically sees a couple of patients per week, depending on the study and where patients are in the cycle. Depending on the complexity of the trial, patients may see the research team only one time or several times over many years. Typically, clinical trial patients are seen in clinic every 2-4 weeks. There are many tasks required before, during and after a research visit to ensure everything runs smoothly, so Clinical Research Coordinators dedicate an average of 5-11 hours of work per patient, per visit. Regardless of patient load, each employee in the Clinical Research department – as well as participating teams across Renown and UNR Med – always step in to help each other out. According to Lisa, the environment is immensely supportive. “We have a program here at Renown to train employees who have never done clinical trials,” said Lisa. “We love seeing people get more engaged with the important work we do, and every department has been great at collaborating with us. Everyone brings a different perspective.” At the end of the study, Lisa gathers all the information and collects notes into a zip drive or paper binder for archiving. The sponsor lets the Clinical Research team, providers and patients know whether they are on the trial drug or on the placebo. The teams use the data gathered during the study to publish a report or present at conferences, promoting the critical research done to better the lives of patients in our community, and potentially, the world. “I appreciate the time everyone gives us to make sure our research is successful,” said Lisa. “It feels great to work together to make a difference, improve healthcare quality and save lives.” Behind-the-Scenes, Yet on the Frontlines The impact of research studies transcends hospital walls, and this can all be attributed to the dedication of our Clinical Research department. The constant collaboration between this team, lab science, medical assistants and providers, cardiology technologists, sonographers, finance teams and our partners at UNR Med is crucial to safeguarding the success of the studies. Devoted to keeping research close to home, Renown and UNR Med teamed up to form the Clinical Research Office (CRO) in 2021. With the strength of northern Nevada's largest not-for-profit health system and Nevada’s first medical school, this team is dedicated to giving our community access to the latest care innovations. “At UNR Med, we are working with students, residents and academic faculty; on the Renown side, we are working with clinicians and community participants,” said Amber Emerson, Manager for Community Outreach and Research Engagement for UNR Med. “Everything we do is data-driven,” added Kristen Gurnea, Manager of Clinical Research for Renown. “Our main goal is to optimize our impact and provide a community benefit for our patients. The scope of our roles in the Clinical Research office is very diverse.” To help meet the growing need locally for healthcare and cutting-edge treatment solutions, the CRO has continued to grow, expanding its research capabilities and helping bring new medications, medical devices and more to patients across northern Nevada and northeastern California. “Once upon a time, our team had only six members; today we have grown to a team of 25,” added Diana Torres, Research Resource Analyst for Renown. “We used to be considered one department, including Medical Education, and we have since branched off into our own cost center. We branched off even further and created a separate Genetics department that runs the Healthy Nevada Project. Throughout this process, the Clinical Research department was always the main point of the umbrella.” “We participate in hospital-wide outreach and marketing, and we feel this has really helped us get the word out about our department,” added Raul Arellano, Research Resource Analyst for Renown. “In fact, we doubled our clinical trial portfolio from last year.” The CRO currently operates over 100 clinical trials locally in cardiology, endocrinology, infectious disease, neurology, pediatric and adult oncology, pediatric sub-specialties and pulmonology. Behind the curtains of in-person research, the CRO is home to several experts who help turn our research studies into a reality, from budgeting and billing to barrier-breaking and building relationships. “I help with barriers patients and Clinical Research Coordinators are facing, building connections and relationships inside and outside of our health system,” said Kristen Gurnea. “I enjoy handling all the supporting pieces that are required for studies to happen.” “My role changes every day,” added Jenna Berger, Administrative Assistant for the CRO at Renown. “Some days, I’ll be helping coordinate patient stipends and going through document management to ensure we have all necessary signatures. Other days, I will be planning events – like Clinical Trials Week – for our department and creating marketing materials and fliers.” “Our day-to-day involves going over anything related to research financials,” added Diana Torres. “We handle sponsor billings, process efficiency and collecting revenue for research contracts, and we collaborate closely with our Finance department and Revenue Integrity in order to accomplish this. It’s important for us to make sure all billing on both the sponsor and patient side is taken care of, especially because patients should never receive a bill for medical services they receive for the trial. A year and a half ago, we started doing budget negotiations for research contracts,” said Diana Torres. “We are proud to help clinical teams with any training they may need on these negotiations as well as billing reviews and allocations.” Seeing patients progress during a study and transform before their eyes inspires the CRO team to continue doing what they do every day. “I’ve been here for many years, first working on the floor as an oncology nurse and transitioning to oncology research in 2005,” said Anna Winchell, Cancer Protocol Nurse for Renown. “I love getting to know the patients and seeing them progress into a healthy lifestyle.” Medical students and residents at UNR Med also play a significant role in the research process, advancing medicine by exploring causes and novel treatments for a wide range of conditions, including HIV, muscular dystrophy, gastrointestinal disorders, infectious diseases and more. Medical research at UNR Med is headed by committed research coordinators, community outreach managers, grants managers, pharmacists and physicians. “I oversee scientific review and help the physicians that come to us for those resources,” said Amil Trujillo-King, Medical Research Coordinator at UNR Med. “I guide medical students in their research protocols and help with different projects to improve research activities for both students and medical residents.” It takes a village to make clinical research happen. Because of that, the ACRO cannot thank the following teams enough for moving mountains for the future of medicine: Renown Health and UNR Med leadership for demonstrating the integrated health system’s commitment to expanding access to clinical research in our community within both the Renown / UNR Med affiliation and Renown active strategic plans. Renown Pharmacy especially Research Clinical Pharmacist Tim Morton, who supports all clinical trial medication dispensing and patient education across all clinical trials at Renown. Accounts Payable for having a huge impact on patient and employee reimbursement. Renown Medical Group for their participating providers, especially in oncology, cardiology, pulmonology, pediatrics, endocrinology and neurology, who are involved in research year after year. Marketing and Communications for helping with printed materials and raising awareness for clinical research at Renown and UNR Med. An Affiliation to Last Through the Ages A collective, shared vision of exploring community health – that is the impetus behind the affiliation between Renown and UNR Med. By leveraging resources across both institutions, the CRO has maximized their impact, giving the people of northern Nevada greater access to new interventions and treatments and promoting an impassioned culture with patients, providers, residents and medical students. “Community-based research always sat well with me,” said Amber Emerson. “As Renown and UNR Med, we have this unique opportunity to shape clinical research here in northern Nevada. We always make sure we present research in a meaningful way that speaks to the work we produce and demonstrates the opportunities we offer. After all, participating in clinical research doesn’t mean our patients are ‘guinea pigs’ – quite the opposite! They are partners in their health care, and we support them through providing access to novel treatments.” “Research is my passion, and my career has spanned broadly from grants administration to study coordination,” added Valerie Smith, Clinical Research Center Administrative Manager at UNR Med. “I am excited to be at the forefront of research frontiers in northern Nevada.” Through robust engagement and collaboration with healthcare providers, department administrators, internal research team members and leadership, the strength of this affiliation is unmeasurable. The CRO’s ultimate goal is to have clinical trials be the standard of care for every condition that Renown and UNR Med treats. Clinical research participation is all about patient autonomy, shared decision-making between patients and their providers and advancing medicine to save lives. From their beginnings as a small group of passionate researchers to their present reality as a leader in the research space in northern Nevada, their efforts do not go unnoticed. “The success of our department is inspiring,” said Amil Trujillo-King. “Renown and UNR Med supports the wellbeing of all employees and contributes directly to the growth of the department.” “When I first joined Renown in Patient Access, I didn’t realize that we had a research department; with a strong healthcare background in my family, I knew I wanted to grow in my career, and our expanding Clinical Research office was that next step,” said Raul Arellano. “With our affiliation with UNR Med, it’s especially inspiring to be able to apply what I learned as a Patient Access Representative to help further outcomes for our patients through managing our finances.” Through their unwavering commitment to research excellence and patient-centered care, the CRO will continue to pave the way for groundbreaking medical discoveries and improved outcomes for patients for years to come. “Fundamentally, we’re working to build a culture of research in our community because we believe it is the right thing to do. Our community deserves to have access to clinical trials and novel care close to home with a dedicated team to support them every step of the way,” closes Kristen Gurnea.
-
¿Qué significa participar en un estudio clínico?
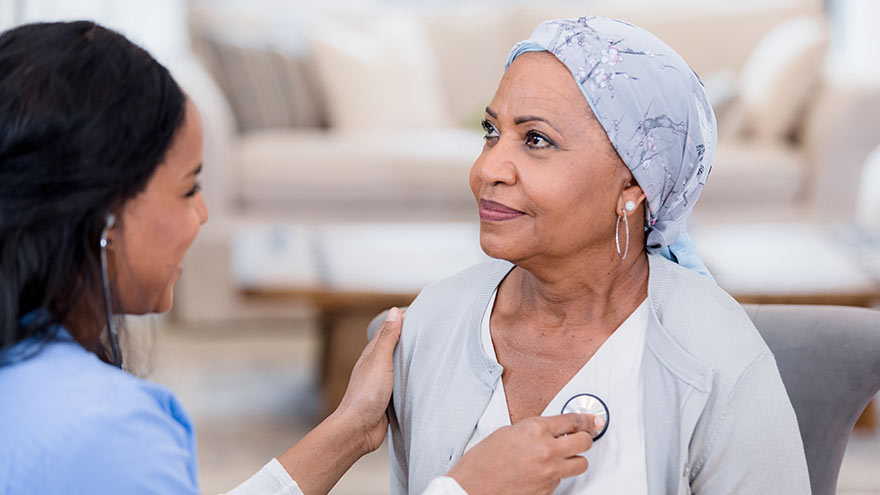
Participating in a clinical trial is voluntary and a personal choice. Clinical trials are research studies that involve people and are an important part of patient care. What is a clinical trial? Clinical trials are research studies that involve people, and they are an important part of patient care. There are several different types of clinical trials; some are designed to understand trends in a disease or identify better ways to diagnose a condition, while others determine if a new treatment is safe and works when treating, improving or preventing a health condition. There are over 400,000 clinical trials currently being conducted in the United States, and even more across the world. This includes health conditions such as heart failure, cancer, Parkinson’s Disease, respiratory conditions like COPD, common infections, cystic fibrosis, and many more. Clinical trials lead the healthcare industry to new discoveries that contribute to reliable and exact care, improving healthcare quality and saving lives. Clinical trials are conducted by a team of researchers, including doctors, pharmacists and clinical research coordinators. These research teams are highly skilled in their specialty areas, often providing traditional patient care and seeing research patients in the same day. These teams are responsible for making sure the clinical trial is completed correctly, and their patients are their top priority. Why should I consider participating in a clinical trial? Participating in a clinical trial is voluntary and a personal choice. There are many reasons why patients decide to get involved in clinical research. While many clinical trials are designed for patients who have a certain health condition, many studies also ask healthy volunteers to contribute in order to compare health outcomes. Clinical trials are also for patients at all different stages of their diagnosis. Depending on the specific study, the patient may receive access to a new cutting-edge treatment before it is widely available. When patients join a clinical trial, the research team becomes a health partner dedicated to their health and well-being. When patients join a clinical trial, they make an informed decision in their healthcare by weighing all available options in addition to routine treatments. Research participants know that they are contributing meaningfully and helping other patients like them. Where can I find more information about clinical trials at Renown Health? Renown Health’s mission is to make a genuine difference in the health and well-being of the communities we serve. Renown’s clinical trial portfolio offers leading care options to patients in northern Nevada, close to home, in a variety of specialties. Contact the Renown Clinical Research Office for more information on clinical trials available to you!
Read More About What Does It Mean to Participate in a Clinical Trial?
-
Gilead Sciences y el Renown Institute for Health Innovation anuncian una colaboración estratégica para promover la comprensión de la esteatohepatitis no alcohólica (EHNA)
Gilead Sciences, Inc. (Nasdaq: GILD) and the Renown Institute for Health Innovation (IHI) today announced a strategic collaboration to collect and analyze genetic and electronic health data that can enhance the understanding of nonalcoholic steatohepatitis (NASH) and potentially inform development of treatment options for the disease. En virtud de los términos del acuerdo de colaboración y licencia, Gilead proporcionará fondos al Renown IHI para secuenciar y analizar el ADN de 15,000 personas que viven con EHNA o con enfermedad de hígado graso no alcohólico (EHGNA), así como de una cohorte de control de 40,000 personas en el estado de Nevada. “Combining the sequencing of protein coding DNA, with extensive electronic health record data will enable a deep analysis of the roles of genetics and environment in NASH incidence and progression,” said John McHutchison, AO, MD, Chief Scientific Officer and Head of Research and Development, Gilead Sciences. “The analysis of these large datasets in collaboration with Renown IHI could help identify genetic variants that impact the risk of developing NASH and thereby advance the discovery and development of new treatments for this disease.” Renown Health es la red de atención médica más completa e integrada de Nevada y mantiene registros médicos electrónicos para 1.02 millones de pacientes registrados. En 2016, Renown Health y el Desert Research Institute crearon el Healthy Nevada Project (HNP), el primer estudio de salud poblacional basado en la comunidad del país. En 2017, el HNP comenzó una sociedad con Helix para aprovechar sus servicios de salud poblacional, la secuenciación Exome+™ y las herramientas de participación del consumidor. The HNP is now an ongoing collaboration between Renown IHI, the Desert Research Institute, a global leader in environmental data and applied research, and Helix, a personal genomics company. HNP combines genetic, environmental, social and clinical data to address individual and community health needs with the goal of improving health across the state and the nation. El HNP actualmente tiene 40,000 participantes. “Combining genetic sequencing with large sets of data can play a critical role in understanding and identifying serious health risks, including diseases like NASH. We are excited to collaborate with Gilead to better understand the condition and its complexities,” said Anthony Slonim, MD., DrPH. “Any genetic variants identified in participants through the collaboration may be shared with the participants for patient care purposes.” About NASH Nonalcoholic steatohepatitis (NASH) is a chronic form of liver disease characterized by excess fat in the liver, inflammation, and liver cell damage. Inflammation and liver cell damage can cause scarring of the liver, or fibrosis, and ultimately lead to cirrhosis or liver cancer. La EHNA es más frecuente en personas con ciertas afecciones, que incluyen obesidad y diabetes tipo 2. There are currently limited approved treatments for patients living with NASH. About Gilead Sciences Gilead Sciences, Inc. is a research-based biopharmaceutical company that discovers, develops and commercializes innovative medicines in areas of unmet medical need. The company strives to transform and simplify care for people with life-threatening illnesses around the world. Gilead opera en más de 35 países alrededor del mundo, con sede central en Foster City, California. Para obtener más información sobre Gilead Sciences, visite el sitio web de la empresa en www.gilead.com. About Renown Health Renown Health is a locally governed and locally owned, not-for-profit integrated healthcare network serving Nevada, Lake Tahoe and northeast California. Renown es uno de los mayores empleadores privados de la región y cuenta con una fuerza laboral de más de 7,000. It comprises three acute care hospitals, a rehabilitation hospital, the area’s most comprehensive medical group and urgent care network, and the region’s largest and only locally owned not-for-profit insurance company, Hometown Health. Renown Health’s commitment has extended beyond traditional health care to include community health and well-being. Renown Health works to improve health care through science, research and genetics; forge community partnerships that improve lives and develop innovative models that are improving health care in Nevada. For more information, visit renown.org. About Helix Helix’s mission is to empower every person to improve their life through DNA. Helix is accelerating the integration of genomic data into clinical care and broadening the impact of large-scale population health programs by providing comprehensive expertise in DNA sequencing, bioinformatics, and individual engagement. Mediante el uso de su ensayo Exome+™ de propiedad exclusiva, un exoma de tipo panel mejorado con más de 300,000 regiones no codificantes informativas, Helix ofrece a los sistemas de salud una solución escalable que permite el descubrimiento de información genética médicamente relevante y que potencialmente puede salvar vidas. Additionally, Helix offers a suite of DNA-powered products for continued individual engagement and discovery. Helix is headquartered in the San Francisco Bay Area and has one of the world’s largest CLIA-certified, CAP-accredited Next Generation Sequencing labs, located in San Diego, California. Obtenga más información en www.helix.com. Helix, the Helix logo, and Exome+ are trademarks of Helix Opco, LLC. Gilead Forward-Looking Statement This press release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that are subject to risks, uncertainties and other factors, including the risk that the parties may not realize the potential benefits of this collaboration, and Gilead may fail to discover, develop and commercialize any product candidates for the treatment of NASH. All statements other than statements of historical fact are statements that could be deemed forward-looking statements. These risks, uncertainties and other factors could cause actual results to differ materially from those referred to in the forward-looking statements. The reader is cautioned not to rely on these forward-looking statements. Estos y otros riesgos se describen en detalle en el Informe Trimestral de Gilead en el Formulario 10-Q para el trimestre que finalizó el 31 de marzo de 2019, presentado ante la Comisión de Bolsa y Valores de los EE. UU. All forward-looking statements are based on information currently available to Gilead, and Gilead assumes no obligation to update any such forward-looking statements. For more information on Gilead Sciences, please visit the company’s website at www.gilead.com, follow Gilead on Twitter (@GileadSciences) or call Gilead Public Affairs at 1-800-GILEAD-5 or 1-650-574-3000. Additional Media Contact: Sung Lee, Investors 650-524-7792 Arran Attridge, Media 650-425-8975
-
Renown Institute Expands Partnership to Offer ELF Testing
Juntos, realizarán pruebas a más de 30,000 participantes del estudio elegibles antes de 2023 para determinar el riesgo de cirrosis y enfermedades relacionadas con el hígado. Renown Institute for Health Innovation (IHI) announced today that the organization and Gilead Sciences, Inc. will be joining forces with Siemens Healthineers to offer the Enhanced Liver Fibrosis (ELF™) Test to people with risks for nonalcoholic steatohepatitis (NASH) enrolled in the Healthy Nevada Project (HNP). The ELF Test will help identify people most at risk for progressing to cirrhosis and liver-related outcomes and allow healthcare providers to intervene before irreparable damage occurs. This noninvasive blood test uses three serum biomarkers to create an ELF score from a predefined algorithm, which can be used by doctors to help evaluate if a patient requires increased medical care and monitoring for their condition. La enfermedad de hígado graso no alcohólico (EHGNA), que incluye la EHNA, es frecuente en Nevada y está subdiagnosticada, y probablemente afecte a más de 500,000 adultos en nuestro estado. If undetected and untreated, NASH can result in liver cirrhosis and may require liver transplantation or lead to death. Hay más de 12,000 personas en la lista de espera para trasplante de hígado en los EE. UU. y esta cifra continúa aumentando debido al incremento en la prevalencia de la EHGNA. “Thanks to important data collected through our Nonalcoholic Steatohepatitis Liver Disease Genome Atlas study, we now know that NASH is prevalent in the state of Nevada,” said Tony Slonim, M.D., DrPH, FACHE, president and CEO of Renown Health. “We are proud to expand our partnership with Gilead and begin working with Siemens Healthineers to improve health of those with liver disease and to take early detection one step further by offering Enhanced Liver Fibrosis, ELF testing for patients of Renown Health. This test provides our team of highly-skilled physicians an advanced, noninvasive method to actively assess dynamic liver fibrosis in study participants and intervene whenever necessary, contributing to a healthier Nevada.” “Gilead believes that noninvasive tests, including the ELF Test, will help improve the experience of people living with NASH. These tests may help to diagnose liver disease, monitor disease progression and evaluate responses to treatment without the requirement for liver biopsy,” said Rob Myers, MD, Vice President, Liver Fibrosis Clinical Research at Gilead Sciences. “The ELF Test has proven itself to be a valuable tool in NASH management and we hope this partnership will further support its use in routine care.” “We are very pleased that NASH patients in the Healthy Nevada Project now have access to the ELF Test which offers clinically useful prognostic information for their condition with the convenience of a simple blood test. Using our advanced laboratory expertise together with Renown IHI and Gilead, we can work towards better understanding of NASH and liver disease in a representative patient population,” said Sebastian Kronmueller, Head of Molecular Diagnostics at Siemens Healthineers. “We launched the Healthy Nevada Project to help people understand more about their health, to identify serious health risks, and to give people access to innovations like the ELF Test, so they can live their best lives,” said Renown’s chief scientific officer, Dr. Joseph Grzymski, who is also a research professor at the Desert Research Institute and principal investigator of the Healthy Nevada Project. Es increíblemente gratificante poder informar hallazgos clínicos para ayudar a nuestros 50,000 participantes voluntarios del estudio y asistir a los proveedores de atención médica a ayudar a sus pacientes”. La provisión de la prueba de ELF se basa en una colaboración estratégica anunciada previamente entre el Renown IHI y Gilead en julio de 2019. Esta sociedad en curso tiene como objetivo recopilar y analizar datos de salud genéticos y electrónicos anónimos de 60,000 participantes del estudio elegibles con el fin de mejorar la comprensión de la EHGNA y la EHNA y para potencialmente orientar el desarrollo de opciones de tratamiento para estas enfermedades. Acerca de la EHGNA y la EHNA La EHGNA es una acumulación de grasa en el hígado en personas que no tienen antecedentes de abuso de alcohol. Es normal que el hígado contenga algo de grasa, pero si más del 5 por ciento del contenido hepático es grasa, se considera hígado graso (esteatosis). NASH is the most severe form of NAFLD in which a person has liver cell damage and inflammation of the liver. Inflammation and liver cell damage can cause fibrosis, or scarring of the liver, and can cause decreased liver function (1). The symptoms of NASH are often silent or non-specific, making it difficult to diagnose. Aproximadamente, un tercio de las personas con EHNA desarrollan cirrosis o daño hepático irreversible (2). About the ELF™ Test The ELF Test is a noninvasive blood test that can quickly identify which patients are at an elevated risk for developing cirrhosis and other liver-related clinical events (LREs). In contrast to standard liver enzyme tests that reflect liver damage that has already occurred, the ELF Test combines three serum direct biomarkers of active fibrosis. The ELF Test algorithm measures each of these biomarkers to create an ELF score, which can be used as an aid to assess the risk for future disease progression. Doctors may use this ELF score to help evaluate if a patient requires increased medical care and monitoring for their condition. Individuals interested in determining their risk for NASH and its progression are encouraged to enroll in the Nonalcoholic Steatohepatitis Liver Disease Genome Atlas study. Those who have already consented and participated in the study will be contacted with more information on how to receive an ELF blood test. Para obtener más información o para inscribirse, comuníquese a la casilla de correo RenownIHI@renown.org o al teléfono (775) 982-6914. The Enhanced Liver Fibrosis (ELF™) Test kit is not available for sale in the U.S. Product availability may vary from country to country and is subject to varying regulatory requirements. In the U.S., the ELF Testing Service is available from Siemens Healthcare Laboratory, LLC (SHL), a CLIA-certified laboratory located in Berkeley, Calif. The ELF Testing Service, including the establishment of performance characteristics, was developed by SHL. The ELF Test has not been cleared or approved by the U.S. Food and Drug Administration. SHL is regulated under CLIA as qualified to perform high complexity testing. The ELF Test is used for clinical purposes and should not be regarded as investigational use only or research use only. About Renown Health Renown Health is the region’s largest, locally owned and governed, not-for-profit integrated healthcare network serving Nevada, Lake Tahoe and northeast California. Con una fuerza laboral diversa de más de 7,000 empleados, Renown ha fomentado una cultura de excelencia, determinación e innovación de larga data. La organización se compone de un centro de urgencias, dos hospitales de cuidados agudos, un hospital infantil, un hospital de rehabilitación, un grupo médico y una red de atención de urgencias y Hometown Health, la compañía de seguros sin fines de lucro más grande de la región y de propiedad local, Hometown Health. Renown’s institute model addresses social determinants of health and includes: Child Health, Behavioral Health & Addiction, Healthy Aging and Health Innovation. Clinical institutes include: Cancer, Heart and Vascular Health, Neurosciences and Robotic Surgery. Actualmente, Renown está inscribiendo participantes en el estudio genético de salud poblacional basado en la comunidad más grande del mundo, el Healthy Nevada Project®. For more information visit, renown.org. About the Renown Institute for Health Innovation Renown Institute for Health Innovation is a collaboration between Renown Health - a locally governed and locally owned, not-for-profit integrated healthcare network serving Nevada, Lake Tahoe and northeast California; and the Desert Research Institute - a recognized world leader in investigating the effects of natural and human-induced environmental change and advancing technologies aimed at assessing a changing planet. Renown IHI research teams are focused on integrating personal healthcare and environmental data with socioeconomic determinants to help Nevada address some of its most complex environmental health problems; while simultaneously expanding the state’s access to leading-edge clinical trials and fostering new connections with biotechnology and pharmaceutical companies. Learn more at healthynv.org. Renown Health es la red de atención médica más completa e integrada de Nevada y mantiene registros médicos electrónicos para 1.02 millones de pacientes registrados. En 2016, Renown Health y el Desert Research Institute crearon el Healthy Nevada Project (HNP), el primer estudio de salud poblacional basado en la comunidad del país. En 2017, el HNP comenzó una sociedad con Helix para aprovechar sus servicios de salud poblacional, la secuenciación Exome+™ y las herramientas de participación del consumidor. The HNP is now an ongoing collaboration between Renown IHI, the Desert Research Institute, a global leader in environmental data and applied research, and Helix, a personal genomics company. HNP combines genetic, environmental, social and clinical data to address individual and community health needs with the goal of improving health across the state and the nation. El HNP actualmente tiene más de 60,000 participantes. For more information, visit healthynv.org. About Siemens Healthineers Siemens Healthineers AG (listed in Frankfurt, Germany: SHL) is shaping the future of Healthcare. As a leading medical technology company headquartered in Erlangen, Germany, Siemens Healthineers enables healthcare providers worldwide through its regional companies to increase value by empowering them on their journey towards expanding precision medicine, transforming care delivery, improving the patient experience, and digitalizing healthcare. Siemens Healthineers is continuously developing its product and service portfolio, with AI-supported applications and digital offerings that play an increasingly important role in the next generation of medical technology. These new applications will enhance the company’s foundation in in-vitro diagnostic, image-guided therapy, and in-vivo diagnostics. Siemens Healthineers also provides a range of services and solutions to enhance healthcare providers ability to provide high-quality, efficient care to patients. En el año fiscal 2020, que finalizó el 30 de septiembre de 2020, Siemens Healthineers, que tiene aproximadamente 54,000 empleados en todo el mundo, generó ingresos por €14.5 mil millones y un EBIT ajustado de €2.2 mil millones. Encontrará más información disponible en www.siemens-healthineers.com.Media Contacto de Siemens Healthineers: Lance LongwellM: 610-448-6341E: lance.longwell@siemens-healthineers.com
Leer más About Renown Institute Expands Partnership to Offer ELF Testing
-
Un estudio demuestra la importancia de garantizar el seguimiento del participante y del proveedor después de obtener el resultado de un examen de detección genético
Lanzada en asociación con el Desert Research Institute, una nueva investigación del proyecto Healthy Nevada Project® determina que un diagnóstico confirmado no siempre produce cambios en el cuidado del paciente. El hecho de entregar a las personas información de salud que pueda cambiar sus vidas no garantiza que las personas, o sus proveedores de atención médica, actuarán en consecuencia. La educación de seguimiento y las conversaciones sobre los planes de cuidado ejecutables con los pacientes y sus médicos son los próximos pasos clave, de acuerdo con la nueva investigación del Healthy Nevada Project. El Healthy Nevada Project es un proyecto de investigación y detección genética que se lanzó en 2016 como una asociación entre DRI y Renown Health. El proyecto ahora tiene más de 50,000 participantes y cuenta con secuenciación genética proporcionada por Helix. Entre septiembre de 2018 y septiembre de 2020, el Healthy Nevada Project notificó exitosamente a 293 participantes que estaban genéticamente en riesgo de desarrollar cáncer de pecho y ovario, síndrome de Lynch o hipercolesterolemia familiar hereditarios, tres afecciones genéticas comunes conocidas colectivamente como afecciones de Nivel 1 de los Centros para el Control y la Prevención de Enfermedades (Centers for Disease Control and Prevention, CDC). En un estudio publicado hoy en Frontiers in Genetics, los científicos del Healthy Nevada Project analizaron qué impacto tuvo el hecho de notificar a un paciente sobre un resultado positivo para una condición de Nivel 1 de los CDC en el cuidado que recibió el paciente en los meses y años posteriores. Según sus resultados, entre los 293 participantes del Healthy Nevada Project que fueron notificados del riesgo genético de desarrollar una afección del Nivel 1 de los CDC, el 71 % de participantes con registros médicos electrónicos compartieron sus hallazgos con los proveedores de atención médica. Sin embargo, solo el 30 % de los registros médicos electrónicos de estos pacientes contenía documentación del diagnóstico genético, y solo el 10 % de los pacientes examinados experimentó un posible cambio en el cuidado después de recibir los resultados de su examen de detección genético. “El Healthy Nevada Project se implementó con un enfoque ‘sin intervención’ en el que los participantes reciben sus hallazgos y deciden con quiénes y cuándo desean compartirlos. Los hallazgos no se agregaron automáticamente a sus registros médicos electrónicos”, explicó el Dr. Gai Elhanan, científico de datos médicos en DRI y coautor del estudio. “Lo que estamos aprendiendo ahora es que para garantizar que los hallazgos genéticos importantes se integren en el régimen de cuidado, es importante que su incorporación en los registros médicos electrónicos forme parte del estudio”. Este estudio se basa en investigaciones anteriores del Healthy Nevada Project publicadas en Nature Medicine. En ellas se demuestra la importancia de realizar exámenes de detección para las afecciones del Nivel 1 de los CDC, las cuales afectan a aproximadamente a 1 de cada 75 personas y se pueden mitigar e incluso se puede evitar que se conviertan en enfermedades cuando se detectan en forma temprana. Este estudio determinó que hasta el 90 % de los casos con afecciones del Nivel 1 de los CDC no son detectados por los proveedores clínicos en las evaluaciones y los exámenes de detección que se realizan durante las visitas de cuidado clínico habitual. Durante el estudio actual, los científicos del Healthy Nevada Project descubrieron que el 19 % de participantes estudiados ya había desarrollado una de las afecciones del Nivel 1 de los CDC y, por lo tanto, podrían haberse beneficiado de una notificación más temprana sobre su afección. El equipo del estudio espera que sus hallazgos estimulen a los residentes de Nevada a obtener pruebas genéticas para estas afecciones relativamente comunes. Incluso si las personas son mayores o ya han sufrido enfermedades relacionadas con estas afecciones, las pruebas también podrían ser beneficiosas para los hermanos, los niños y los nietos que también pueden estar en riesgo y que posteriormente podrían someterse a exámenes de detección en caso de un hallazgo positivo. El equipo del estudio también incita a informar a los proveedores de atención médica sobre la importancia de incorporar diagnósticos genéticos en las recomendaciones farmacéuticas (por ejemplo, para la hipercolesterolemia familiar) y de tratamiento a los pacientes. “Como resultado de este análisis, los médicos de Renown Health y los investigadores del Healthy Nevada Project han logrado cambios significativos, lo que incluye la obtención del consentimiento informado de los participantes para incorporar hallazgos positivos de sus informes genéticos directamente en su registro médico electrónico”, explicó Daniel Kiser, M.S., científico auxiliar de investigación de ciencia de datos en DRI y coautor del estudio. “Esto ayudará tanto a los participantes como a sus proveedores de cuidado clínico, y a todo el estado a maximizar los beneficios a largo plazo de los exámenes de detección genéticos voluntarios basados en la población del Healthy Nevada Project”. Información adicional: El texto completo del estudio, Incomplete Penetrance of Population-Based Genetic Screening Results in Electronic Health Record, está disponible en Frontiers in Genetics: https://www.frontiersin.org/articles/10.3389/fgene.2022.866169/full?&utm_source=Email_to_authors_&utm_medium=Email&utm_content=T1_11.5e1_author&utm_campaign=Email_publication&field=&journalName=Frontiers_in_Genetics&id=866169. Este proyecto fue financiado por Renown Health, fundación de Renown Health y la Oficina de Desarrollo Económico del Gobernador de Nevada. Entre los autores del estudio se incluyen Gai Elhanan (DRI), Daniel Kiser (DRI), Iva Neveux (DRI), Shaun Dabe (Renown Health), Alexander Bolze (Helix), William Metcalf (DRI), James Lu (Helix) y Joseph Grzymski (DRI/Renown Health). Para obtener más información sobre el Healthy Nevada Project® o para solicitar un examen de detección genético, visite: https://healthynv.org/ Acerca del DRI El Desert Research Institute (DRI) es un reconocido líder mundial en investigación ambiental básica y aplicada. Comprometidos con la excelencia e integridad científicas, el cuerpo docente del DRI, los estudiantes que trabajan juntos y el personal han desarrollado conocimientos científicos y tecnologías innovadoras en proyectos de investigación en todo el mundo. Desde 1959, la investigación de DRI ha profundizado el conocimiento científico sobre temas que van desde el impacto de los seres humanos en el medioambiente hasta el impacto del medioambiente en los seres humanos. Los hallazgos científicos impactantes y las soluciones inspiradoras del DRI apoyan la economía diversa de Nevada, proporcionan oportunidades educativas basadas en la ciencia e informan a legisladores, líderes empresariales y miembros de la comunidad. El DRI tiene campus en Las Vegas y Reno y funciona como el grupo de investigación sin fines de lucro del Sistema de Educación Superior de Nevada. Para obtener más información, visite www.dri.edu. Acerca de Renown Health Renown Health es la red de atención médica integrada de administración local y sin fines de lucro más grande de la región, que presta servicios a Nevada, Lake Tahoe y el noreste de California. Con una fuerza laboral diversa de más de 7,000 empleados, Renown ha fomentado una cultura de excelencia, determinación e innovación de larga data. La organización se compone de un centro de urgencias, dos hospitales de cuidados agudos, un hospital infantil, un hospital de rehabilitación, un grupo médico y una red de atención de urgencias y Hometown Health, la compañía de seguros sin fines de lucro más grande de la región y de propiedad local, Hometown Health. Actualmente, Renown está inscribiendo participantes en el estudio genético de salud poblacional basado en la comunidad más grande del mundo, el Healthy Nevada Project®. Visite renown.org para obtener más información. Acerca de Helix Helix es la compañía líder en genómica y vigilancia viral de la población cuyas operaciones se basan en el cuidado clínico, la investigación y el análisis de datos. Helix permite que los sistemas de salud, las empresas de ciencias biológicas, los pagadores y los socios gubernamentales aceleren la integración de los datos genómicos en el cuidado médico del paciente y en la toma de decisiones de salud pública. Obtenga más información en www.helix.com.
-
Could Private Well Water Unlock Health Insights?
The Healthy NV Project® launches a new study to identify how private well water could impact the health of those living in the household. You use your faucet to wash your hands, make coffee and fill your water bottle. Sin embargo, para las aproximadamente 41,000 personas en los condados de Washoe y Churchill que dependen de agua de pozos privados, es posible que el H2O contenga contaminantes no detectados que podrían estar afectando su salud. The Healthy Nevada Project®, with support from the National Institutes of Health (NIH), is launching a new study to discover how private well water quality impacts the health of well owners. By providing free water testing kits to interested Healthy Nevada Project® participants, researchers will collect samples and give results back to well owners, along with resources for potential treatment options. Data from previous studies in Nevada show elevated concentrations of heavy metals in some private, household wells. Since water from household wells is not monitored for quality by government agencies, well water testing helps ensure water does not contain dangerous levels of heavy metals, such as lead, which can lead to adverse health impacts. Those with a household well interested in receiving a free water testing kit must be enrolled in the Healthy Nevada Project’s population genetic screening study and consent to be a part of further research. People interested in joining the study can simply sign up to receive a genetic spit test kit in the mail or join the waitlist to be notified when in-person testing resumes. “The goals of the Healthy Nevada project are to improve population health and better understand processes that increase disease risks, such as cancer. In this study, we engage with our study participants and inform them about the impact of the environment on their health, while researching environmental contaminants that may elevate cancer risk,” said Joseph Grzymski, Ph.D., research professor at DRI, principal investigator of the Healthy Nevada Project® and chief scientific officer for Renown Health. “En un tiempo en el que las personas pasan más tiempo en casa, nos complace lanzar este estudio multidisciplinario que indaga en los posibles impactos en la salud que puede tener la fuente de agua de un hogar”. “Mientras el Healthy Nevada Project continúa sirviendo a más de 54,000 voluntarios de investigación, nos enorgullece proporcionar información útil a los participantes para que puedan hacer mejoras transformadoras en su entorno doméstico”, dijo Tony Slonim, D.M., DrPH, FACHE, presidente y director ejecutivo de Renown Health y codirector del Healthy Nevada Project. “This allows every person with well water in Nevada, to have important information, at no charge, to help live healthier and better lives and to protect their family’s health.” The Healthy Nevada Project® is the fastest-enrolling genetic study in the country. The Project is also the first of its kind to return clinical results to study volunteers, which means participants can learn their genetic risks tied to heart disease and certain cancers, as well as lifestyle changes that could potentially help reduce their risk and prevent disease. To enroll in the Healthy Nevada Project, please visit healthynv.org. Renown Institute for Health Innovation is a collaboration between Renown Health - a locally governed and locally owned, not-for-profit integrated healthcare network serving Nevada, Lake Tahoe and northeast California; and the Desert Research Institute - a recognized world leader in investigating the effects of natural and human-induced environmental change and advancing technologies aimed at assessing a changing planet. Renown IHI research teams are focused on integrating personal healthcare and environmental data with socioeconomic determinants to help Nevada address some of its most complex environmental health problems; while simultaneously expanding the state’s access to leading-edge clinical trials and fostering new connections with biotechnology and pharmaceutical companies. Learn more at healthynv.org.
Leer más About ¿Podría el agua de pozos privados ofrecer información sobre la salud?
-
Departamento destacado: Farmacia
When it comes to each patient’s healthcare journey, medication is often a key chapter. After all, medication is one of the most common treatment methods to help patients on the road back to health. In 2023, 4.83 billion prescriptions were filled in the U.S., and with this number only anticipated to rise annually, having an expert pharmacy team on your side to make certain you are prescribed the ideal dosage to treat your condition, prepare your prescriptions on time and help you manage your medications responsibly is important. Fortunately, at Renown Health, we have best-in-class inpatient and outpatient pharmacy teams to fill both prescriptions and promises for excellent care. Renown Pharmacy plays a vital role in helping us foster a health system that prioritizes patient well-being above all else. This department exemplifies the impact that a unified, expert pharmacy team can have on patient outcomes now and in the future. The Masters of Medication Spanning three hospitals plus ambulatory locations across the health system, Renown’s growing pharmacy team – full of dedicated pharmacists, pharmacy technicians and even medical assistants – manages medications in a wide variety of patient settings, touching nearly every aspect of the healthcare continuum: Outpatient Retail Pharmacies Renown Regional Medical Center – 75 Pringle Way The Healthcare Center – 21 Locust Street Renown South Meadows Medical Center – 10101 Double R Blvd Inpatient Pharmacies Renown Rehabilitation Hospital Renown Regional Medical Center (including Renown Children’s Hospital) Renown South Meadows Medical Center COMING SOON: Conrad Breast Center Pharmacy (in honor of Kristina Ferrari) in the Specialty Care Center at Renown South Meadows Ambulatory Pharmacies Anticoagulation Services – Institute for Heart & Vascular Health (IHVH) Pharmacotherapy Program – IHVH and Renown Medical Group Locations Congestive Heart Failure Pharmacotherapy Program – Center for Advanced Medicine B at Renown Regional Chronic Obstructive Pulmonary Disease (COPD) Pharmacotherapy Program – Renown South Meadows Endocrinology Pharmacotherapy Program – Renown South Meadows Additional Pharmacy Programs Medical Reconciliation Pharmacy Residency Clinical pharmacists at Renown bridge the gap between medicine and compassionate support, making sure each patient receives personalized care one prescription at a time. “There are various roles pharmacists play within Renown,” said Clarissa Munoz, Clinical Pharmacist in the Renown Regional Inpatient Pharmacy. “Staff pharmacists work diligently to ensure correct medications are dispensed, and if compounded, make sure they were prepared properly. They also work hard to answer medication messages and phone calls, help verify orders and make sure ode trays/RSI kits are appropriately stocked and ready when needed. Clinical pharmacists work from satellite pharmacies on the floor and focus on reviewing patient charts and aim to provide additional interventions to the providers to optimize treatment strategies. We also serve as a resource for nursing staff and help answer medication questions.” “My role in the pharmacy is pretty expansive,” added Chanelle Ajimura, Clinical Pharmacist in the Renown Regional Outpatient Pharmacy. “I maintain inventory to confirm patients can receive their medications in a timely manner both for our discharge and retail patients while balancing the Meds to Beds program, which offers medication delivery to the bedside and bedside medication counseling; collaborating with an interdisciplinary team to find the most affordable price for patients; and verifying that the dose, strength, indication, etc. is appropriate for the patient from start to finish.” “In the pharmacy, I make sure patients are receiving appropriate drug therapy by checking for major drug interactions and ensuring appropriate dosing,” added Courtney Church, Clinical Pharmacist in the Renown Regional Outpatient Pharmacy. “I also make recommendations to providers so patients can get cost-effective therapy.” Our pharmacy technicians work behind-the-scenes ensuring efficient medication management, making a difference in the lives of patients every day. “A pharmacy technician is responsible for making sure the patient gets their medications on time and at the lowest price possible,” said Nate Graham, Pharmacy Technician in the Renown Regional Outpatient Pharmacy. “This is done by working with patients, insurance companies and case workers. We fill prescriptions, enter prescriptions into our system, receive and send orders for medications and maintain a clean pharmacy with an accurate inventory.” “We do a variety of things; the task people probably know the most is counting out the medications and putting them in the amber vials,” added Rachel Vallin, Pharmacy Technician in the Renown Regional Outpatient Pharmacy. “We also help patients at the front of the pharmacy, ring out their prescriptions, answer some basic questions (deferring to a pharmacist as necessary) and billing insurance. Meds to Beds is my favorite part because I feel the most involved. I take medications to patients who are discharging up to their hospital rooms so they have it with them when they leave.” “As a technician, I confirm that all medications of new admissions are available in our machines prior to admitting and then maintain stock during each patient’s stay,” added Tammara Axtman, Pharmacy Technician at Renown Rehabilitation Hospital. "I also assist our nurses when needed in regard to any of their questions with both EPIC and Omnicell.” Our pharmacy team is also on the move all across our health system, thanks to our Ambulatory Pharmacy programs. For patients experiencing a serious heart, lung, or endocrine condition that requires ongoing drug therapy maintenance and guidance, our ambulatory pharmacies step in to carefully monitor how their medications impact their health and well-being. “Our role as pharmacists in this department is non-traditional because we actually see patients in the exam rooms face-to-face,” said Cory Lankford, Ambulatory Care Clinical Pharmacist for Renown’s Anticoagulation Services. “We modify their medication regimens and drug recommendations under collaborative practice agreements.” “Because our role is so unique, we have a lot of opportunities to make a positive impact on patients,” added Janeen Abe, Ambulatory Care Clinical Pharmacist for Renown’s Anticoagulation Services. “We do a lot of direct patient interaction, including counseling patients on their medications and helping them navigate through their disease state.” “As a medical assistant in this department, we’re called the patient ‘liaisons’ to orchestrate who they should go to whether it’s a nurse, a provider or a pharmacist,” added Kiara Scruggs, Medical Assistant for Renown’s Anticoagulation Services. “We look at each patient’s medications and help with the Warfarin blood thinner monitor. We get to do a lot with patients." A key resource within the Pharmacy department and the emergency admission process, our Medical Reconciliation ("Med Rec") team stays on top of each patient's medication records. By ensuring each medication regimen is accurately reflected in each patient's chart and that patients continue to take their at-home medications while admitted to the hospital, this team provides vital insight into medications that could be a contributing factor to each patient's symptoms, including drug interactions. “Our medication reconciliation pharmacy technician team are true detectives,” said Heather Townsend, Clinical Pharmacy Supervisor. “When a patient arrives to the hospital, Med Rec works with patients, families, caregivers and outpatient pharmacies to compile a list of medications the patient has been taking a home. This list is used to make sure medications are not contributing to the patient’s symptoms and to assure medications are continued throughout the hospital stay. The addition of the medication reconciliation team has been one of the greatest advancements in medication safety.” “As a Med Rec Tech, we interview patients and family members and call pharmacies, skilled nursing facilities, etc. to obtain an accurate and complete medication list/history to outline what the patient is currently taking on a daily basis,” added Kara McGee, Medical Reconciliation Pharmacy Technician. “We make sure that we document the correct medication, dose, route, frequency and directions. This information is crucial because the nurses, pharmacists and physicians look at our work to figure out if any medications are contributing to the patient's health condition, and for the continuation of home medications on admission.” “Even though the Med Rec Tech might seem small in the hospital realm, it is very vital for patient information and beneficial to the patient's health,” added Brizza Villafan, Medical Reconciliation Pharmacy Technician. “There is never a dull moment in this work.” No matter the diagnosis, having Renown Pharmacy as an integral part of your healthcare team is a win-win situation for both you and them: you receive access to medication to help you heal, delivered to you with precision and care, and the pharmacy team has the opportunity to care for you and make a positive impact, a role they take seriously.
-
Name-Brand Medication vs. Generic: What's the Difference?
Most prescriptions meds are available in generic form. Find out the similarities and differences between the two and how to determine whether a generic is right for you. Approximately 80 percent of prescriptions sold today are generics. If you’re taking a prescription medication, chances are it’s a generic form of the brand-name drug. But are you getting the same quality in a generic medication? Do generics measure up? The answer in most cases is yes — generics, just like branded products, are regulated by the Food and Drug Administration. “To have a generic product approved by the FDA, the generic manufacturer must prove that its product is bioequivalent to the branded product,” explains Adam Porath, PharmD, BCPS AQ-Cardiology, BCACP and Vice President of Pharmacy Services. Basically, it has to function the same. “Generic products are extremely well tolerated and will provide the same results as using a branded product,” Porath says. Here’s how generics are the same as name-brand prescriptions: Generic products contain the same active ingredients. They produce the same desired clinical effect and accompanying side effects. Generics come in the same form as their branded counterparts: pill, liquid or inhaler, for example. Release into the bloodstream matches the name brand in timing and strength. Here’s how they differ: Generics generally cost less. Federal law requires generics have different names and look different: shape, size, markings and color. Generics contain different inactive ingredients, like binders, fillers and artificial colors. Different side effects with generics can usually be attributed to these additions. Why do generics cost less? When pharmaceutical companies develop a new drug, they are paying for research, development, clinical studies, marketing — in some cases it can cost more than $800 million and take 10 to 15 years to develop a new drug. “The manufacturers of branded medication products have to recoup their research and development costs,” Porath says. So companies are granted a limited patent to sell their drug without the competition of generic counterparts. “When patent exclusivity ends, the market is open for any generic manufacturer to make a competing product with FDA approval.” Without the same startup costs, companies can sell generics at 80 to 85 percent less. And because more than one company can produce the same generics, competition drives prices even lower.
Read More About Name-Brand Medication vs. Generic: What's the Difference?
-
Getting to the HEART of Research
In February, we think about hearts not just in honor of Valentine’s Day but because it is American Heart Association Month. This is a great reminder to focus on our personal cardiovascular health. Renown Health helps patients think about their heart health with our world-class providers and cutting-edge treatments through our Cardiovascular Clinical Trials. “Research serves a vital role in the future care of cardiovascular diseases. Being involved in research will help our medical community to further discover new treatment plans in our quest for life preservation and extension,” Dr. Thomas To, Cardiologist and Researcher at Renown Health. For example, let’s talk about atherosclerosis. When our hearts are healthy, they are a strong muscle that pumps our oxygen-rich blood through our coronary arteries. Over time, cholesterol and fats can build up in our arteries. This is a condition known as atherosclerosis. This type of plaque buildup in the arteries can lead to a heart attack or stroke if not properly managed. If you are experiencing chest pain or discomfort, shortness of breath or pain in areas of the upper body, these can be the warning signs of a heart attack, and you should call 911. One contributing factor to atherosclerosis is elevated lipoprotein(a) levels and the accumulation of cholesterol in the arteries, which increases the likelihood of a heart attack or stroke. Lipoprotein(a) is tested separately from the standard panel that is completed for cholesterol management, and while your total cholesterol levels may be in a healthy range, lipoprotein(a) levels can still be elevated. "Increasingly we are realizing that lipoprotein(a) levels can be used as an important assessment in more carefully delineating an individual's risk of future cardiovascular events and treatment targets" said Dr. Michael Bloch, Lipid Specialist and Researcher at Renown Institute for Heart and Vascular Health. While it is clear that elevated lipoprotein(a) contributes to atherosclerosis, there are currently no approved medications for reducing cardiovascular disease risk through reducing lipoprotein(a) levels. This is why Renown Health’s Research Office is proud to offer a phase III clinical trial, called the OCEAN(a) study, to our patients with elevated lipoprotein(a) levels as a care option for management of their heart disease risk. Our teams of expert providers and researchers are here to support you on your healthcare journey. “I am thrilled to be able to be part of this study and bring opportunities like this to our patients. The highlight of my day is getting to hear life stories from my patients during our study visits,” Lisa Preciado, Primary Clinical Research Coordinator for the OCEAN(a) study said. Join us in raising awareness around American Heart Month by talking to your provider about lipoprotein(a) at your next appointment. At Renown Health, our goal is to make it easy for patients to access clinical research as a care opportunity where patients can access a variety of standard care treatment options for their health condition or choose to participate in a clinical trial. For more information about clinical trial opportunities available to you or to ask any questions, contact the Renown Research Office at Renown-CRD@renown.org or 775-982-3646.
-
Multiple Sclerosis Research Opportunities in Northern Nevada
There are nearly 1 million adults living with MS in the United States alone. For comparison, that is roughly the entire population of the Reno/Sparks and Las Vegas areas combined. MS is a neurological autoimmune condition which means that the immune system of patients with MS attacks the body’s myelin, a protective substance that covers your nerves. When this happens, the unprotected nerves can be damaged. Patients with MS may experience many different symptoms ranging from mild to severe, such as mobility and vision problems, fatigue and difficulty thinking. MS is usually diagnosed between the ages of 20-50, but late onset MS can occur in patients over 50 years old. While there is no cure for MS, there are effective treatments that can help reduce the burden of patients’ symptoms and create a positive quality of life. At Renown Health, we have joined the fight against MS through a partnership between advanced neurology programs and providers and our research office. We are proud to offer newly diagnosed MS patients the opportunity to choose between standard care treatment options or participating in an open label clinical trial, the AGNOS study. This study is assessing the impact of a new medication, ofatumumab, as the first disease modifying therapy participants receive for managing relapsing remitting MS, the most common form of MS.
Read More About Multiple Sclerosis Research Opportunities in Northern Nevada
-
Sobreviviente del cáncer de ovario cuenta cómo decidió probar con ensayos clínicos
While there used to be three basic treatment options for cancer -- surgery, radiation and chemotherapy, or a combination of the three -- there's a fourth option: clinical trials. Here, a Renown patient shares her successful battle with ovarian cancer, aided by a clinical trial. Shari Flamm's battle with ovarian cancer began in 2011. She was experiencing prolonged bleeding, irregular thyroid levels and anemia and was scheduled to undergo a hysterectomy. Before the surgery, her gynecologist ran routine tests to check for cancer as a precautionary measure. All tests were negative for cancer, expect her CA 125 test. A CA 125 test measures the amount of the protein CA 125 (cancer antigen 125) in the blood. In some cases, a CA 125 test may be used to look for early signs of ovarian cancer in women with a very high risk of the disease. In most laboratories, the normal level is 0 to 35 units/ml. Flamm's CA 125 level was 121. As Flamm can attest, early diagnosis played a key role in her battle with ovarian cancer. September is Gynecologic Cancer and Ovarian Cancer Awareness Month – an important time to learn the signs, symptoms and risk factors of this type of cancer so your doctor can diagnosis the disease as early as possible. Ovarian Cancer: Round One Despite the elevated CA 125 results, her doctor recommended they move forward with the hysterectomy. But when surgery began, doctors discovered a mass. She had stage 4 cancer. The procedure was halted, the mass was biopsied and she was immediately seen by Dr. Peter Lim of the The Center of Hope. Following diagnosis, Flamm underwent surgery with Dr. Lim to remove the cancer, which had spread to part of diaphragm, spleen, colon and other organs. Three months after surgery, Flamm had recovered enough to start six rounds of chemotherapy in her hometown of Carson City. She continued working at a doctor's office during her treatment, and was grateful for Dr. Lim’s ability to co-manage her care so she could stay close to work and family. “To me, chemo was the scariest part because I didn’t like feeling sick,” Flamm says. Thankfully, her body responded well to the treatments and she was back to the things she loved. “I stated working out at the gym, even if it was only for 10 minutes,” she says. She also stayed positive by spending time with her grandchildren, attending a San Jose Sharks hockey game, going for walks and enjoying concerts. Ovarian Cancer: Round Two In November 2014, Flamm had a cancer check-up. That’s when doctors discovered three cancerous tumors. For this round, Flamm choose another treatment option -- clinical trials at Renown Institute for Cancer. Clinical trials are the studies that test whether drugs work, and inform doctors' decisions about how to treat their patients. Flamm participated in a clinical trial that featured oral-targeted therapy stronger than IV chemotherapy. The hope was for the drug to shrink her tumors, however the result was stabilization -- meaning the lumps weren’t growing or spreading. The best part of the clinical trial, Flamm says, was the constant monitoring. Between the CT scans every six weeks, a heart scan every three months and monthly doctor visits, she was confident that if the cancer started growing or spreading, her healthcare team would catch it right away. For Flamm, the benefits of the clinical trial included less hair loss, less fatigue and more time to focus on what’s important in her life -- her family. “I decided I wasn’t going to be that sick grandma on the couch with cancer,” Flamm says. After taking the oral medication for one year, Flamm developed a rash and discontinued treatment due to discomfort. Clinical Trials, Setbacks and Survival In June 2016, two of the three tumors began to grow and had to be surgically removed. Despite the setback, Flamm was determined to maintain a positive outlook. "You have to stay positive because cancer feeds off anger, depression and stress," Flamm says. Flamm was released to go home with clear margins, meaning the tumors were removed and are surrounded by a rim of normal tissue that does not have cancerous cells. Flamm says her outlook on life has changed drastically since her first cancer diagnosis. “Your whole mentality changes when cancer disturbs your life," Flann says. "The things that weren’t important, are now ever so important. I’m a lot calmer now,” Flamm says.
Read More About Ovarian Cancer Survivor Shares Decision to Try Clinical Trial
-
Use Caution: Mixing Over-the-Counter Medications Can Be Harmful
When you’re too sick to go to work but not sick enough for a doctor’s visit, over-the-counter medicines are a welcome relief to help alleviate that fever, runny nose or allergies. But because those medicines aren’t signed off on or managed by your doctor and pharmacist, you must be especially mindful of what you put into your body. Whenever you pop a pill, you want to ensure you’re taking the correct dosage, waiting the right amount of time before taking another dose and not mixing certain medicines. Too Much Tylenol/Acetaminophen Tylenol — or acetaminophen — is a popular pain reliever for many, but too much can be bad for your liver. “Our bodies have a finite ability to metabolize Tylenol,” says Andy Wright, clinical pharmacist at Renown Rehabilitation Hospital. “When too much builds up in the liver, it becomes toxic. In patients with medical conditions like cirrhosis of the liver or hepatitis, this could be disastrous.” Remember, acetaminophen is in more than just Tylenol and generic pain relievers. You may also see acetaminophen in flu, cold and cough medicines, like Nyquil, and some prescription medications including Norco and Percocet. Keep a list of the medications you take, and limit daily acetaminophen use to 3,000 mg per day. When you’re scanning medicine bottle contents, remember acetaminophen is also referred to as APAP, AC, acetam or paracetamol. Mixing Painkillers When you’re dealing with pain and not getting any relief, taking a different medication may seem like the easy solution. Maybe you take some Aleve — a form of naproxen — for a headache, but it isn’t working, so you switch to Motrin, an over-the-counter form of ibuprofen. Not a smart idea. Ibuprofen and naproxen along with aspirin are known as nonsteroidal anti-inflammatory drugs (NSAIDS). Because these medicines work in similar ways, they should never be combined or used in larger doses or more frequently than directed. Otherwise your risk of side effects can increase, which range from mild nausea to severe gastrointestinal bleeding. It’s also important to consider your family history when taking NSAIDs because, “recent studies have shown NSAIDs may have greater cardiovascular risks for people taking blood thinners or those with hypertension,” explains Andy. “A good example is ibuprofen: It has a relatively low gastrointestinal bleed risk while it has a moderate to high cardiovascular risk. The opposite is true for naproxen.” Rather than experimenting with multiple medicines, figure out which drug works best for you. You may find muscle soreness improves with aspirin, whereas when a headache hits, naproxen is best. Keep in mind that these medications aren’t always best for everyone in the family. “Aspirin in children and teens is not recommended unless under the supervision of a doctor,” Andy says. And pregnant and lactating women should generally avoid NSAIDS due to risk of birth defects and bleeding. “In both of these cases, acetaminophen or Tylenol are preferred but only if approved by an OB/GYN.” Fighting Allergies Over-the-counter antihistamines like Claritin, Zyrtec and Allegra have made fighting itchy eyes and runny noses a little easier. But these daily medicines — when taken inappropriately or in the wrong combinations — can also have an adverse effect. Similar to acetaminophen, you need to watch for antihistamines in other products. Sleep aids — like Tylenol PM and Unisom — commonly use an antihistamine known as diphenhydramine, which may increase your risk of overdose. “Combining antihistamines, or overdosing, can cause many adverse effects including dry mouth, blurred vision — even arrhythmias,” Andy says. “Only take these medications on their own.” If you’re still struggling with symptoms, you can talk to your doctor about adding an over-the-counter nasal steroid. Andy confirms the importance of closely following the directions listed on antihistamine (and all medicine) bottles. He has seen extended release nasal decongestants cause significant arrhythmias requiring medical care after a patient took the medicine with warm fluids. “The decongestant in question is designed to slowly release, but it can dissolve suddenly in the presence of warm liquids like coffee,” Andy explains. “This can cause the pill to deliver 12 to 24 hours of medication all at once.” Taking an Antidiarrheal with Calcium Calcium supplements and antidiarrheal medicines are another harmful combination. Calcium firms up your stool, but if taken with an antidiarrheal, can cause severe constipation. If you need to take an antidiarrheal, take a break from your calcium for a few days until you’re back to normal. Another consideration when taking calcium supplements or calcium-based antacids is gas. “I’ve had several patients report cases of excessive gas using Tums or calcium carbonate-based supplements.” Andy suggests instead “trying Maalox or Mylanta for indigestion and Citracal as a supplement.” Talk with Your Doctor or Pharmacist About Your Medications If over-the-counter drugs aren’t providing the relief you need, it’s time to see your doctor. And remember, for your safety it is important to keep your doctor and pharmacist up-to-date with any medications — prescribed or over-the-counter — that you are taking.
Read More About Use Caution: Mixing Over-the-Counter Medications Can Be Harmful