Buscar
-
Research Shows Genetic Approaches to Breast Cancer Screenings Yield More Accurate Results
Clinical researchers with the Healthy Nevada Project co-author research paper with findings that emphasize the need for a comprehensive approach to breast cancer risk assessment – including a focus on genetic medicine – to help ensure that individuals at high risk are identified and supported proactively rather than reactively. Breast cancer is a leading cause of cancer death among women in the United States. According to the American Cancer Society, about 1 in 8 women will develop breast cancer and about 1 in 39 women will die from breast cancer. Breast cancer is associated with increased age, hereditary factors, obesity, and alcohol use. Since 1990, breast cancer death rates have declined progressively due to advancements in treatment and detection. In Nevada there are an estimated 2,310 new breast cancer cases a year, and genetic mutations such as in the genes BRCA1 or BRCA2 remain a top risk factor for this prevalent disease. Recognizing the urgency for progress in breast cancer research, a collaborative effort between physicians, advanced practice providers and scientists from the Healthy Nevada Project® (HNP) and Helix have unveiled groundbreaking research. This study explores how genetic screenings are a necessary supplement to traditional testing methods, together offering more accurate insights into a patient's likelihood of developing breast cancer in the future. HNP is operated by Renown Genomic Medicine and the Institute for Health Innovation and is one of the largest community-based population health studies in the country. Their team works in collaboration with Helix, a leader in precision health that delivers comprehensive genomic solutions. Together, this dynamic partnership aims to understand breast cancer risk factors and pave the way for more effective preventative measures. The combined research team studied 25,591 female HNP participants to evaluate the performance of different genetic screening approaches to identify women at high risk of breast cancer. The results of this research suggest that a combined monogenic, or single-gene, and polygenic, or multi-gene, approach to breast cancer screenings helped produce more accurate results and more closely identify study participants who have a high genetic risk of developing the disease. "Based on this research, we are advocating a shift in approach which would improve breast cancer risk assessment through a combination of effective family history ascertainment and genetic screening,” said Joseph Grzymski, PhD, principal investigator of the Healthy Nevada Project, research professor at the University of Nevada, Reno School of Medicine and co-author of the breast cancer research paper. “This tailored approach, founded on the assessment of individual genetic risk, not only intends to elevate patient well-being but also will improve efficiency and equity in healthcare." Complementing the team’s research on leveraging genetics to identify women at low genetic risk of breast cancer that could safely defer mammogram screenings by five to 10 years that was released in late 2023 in JAMA Oncology, the study suggests that incorporating genetic information can assist in personalizing breast cancer screenings and optimizing the use of screening resources. "Existing disparities persist across various facets of breast cancer screening and treatment; however, genetic screening is clearly a powerful tool to help facilitate early intervention for those at higher risk,” said Jamie Schnell Blitstein, APRN, a primary care nurse practitioner at Renown Health and co-author of the breast cancer research paper. “By placing a heightened focus on risk, we underscore the pivotal role of preventative breast cancer screening.” Despite the availability of effective methods for early screening, co-authors of this research found that 78 percent of women with a family history of breast cancer had their risk ascertained only after a breast cancer diagnosis. The findings emphasize the need for a comprehensive approach to breast cancer risk assessment – including a focus on genetic medicine – to help ensure that individuals at high risk are identified and supported proactively rather than reactively. “These findings that can profoundly impact how healthcare is delivered were only made possible by all the participants who were willing to consent to research,” said Alex Bolze, PhD from Helix and co-author of the publication. “Broad-scale collaboration projects like these between Renown Health and UNR that engage large populations where participants share both their genetic information as well as electronic health records drive advancements in preventative medicine, as well as fundamental biological research.” The research paper was officially accepted on Jan. 29, 2024, and will be published by Elsevier, Inc. on behalf of the American College of Medical Genetics and Genomics. The contents of the paper will appear in the international journal Genetics in Medicine Open. Read the full article by visiting sciencedirect.com. The Healthy Nevada Project is currently recruiting new study participants. Free to all Nevadans with a saliva sample or blood draw, participants and their referring providers receive access to whole-exome sequencing and clinical grade results that help provide insight into their unique genetic risks tied to heart disease and certain cancers. If you are interested in enrolling in the study, schedule a Virtual Consent Appointment through MyChart or contact the Renown Institute for Health Innovation at RenownIHI@renown.org or (775) 982-6914 to be connected to a Genomic Representative. About Renown Health Renown Health is the region’s largest, not-for-profit integrated healthcare network serving Nevada, Lake Tahoe and northeast California. Con una fuerza laboral diversa de más de 7,000 empleados, Renown ha fomentado una cultura de excelencia, determinación e innovación de larga data. La organización se compone de un centro de urgencias, dos hospitales de cuidados agudos, un hospital infantil, un hospital de rehabilitación, un grupo médico y una red de atención de urgencias y Hometown Health, la compañía de seguros sin fines de lucro más grande de la región y de propiedad local, Hometown Health. Renown is currently enrolling participants in the largest community-based genetic population health study, the Healthy Nevada Project®. To join the Renown Health team, visit renown.org/careers. Acerca de Helix Helix es la compañía líder en genómica y vigilancia viral de la población cuyas operaciones se basan en el cuidado clínico, la investigación y el análisis de datos. Helix enables health systems, life sciences companies, payers, and government partners to accelerate the integration of genomic data into patient care and public health decision-making. Learn more at helix.com.
-
Gilead Sciences y el Renown Institute for Health Innovation anuncian una colaboración estratégica para promover la comprensión de la esteatohepatitis no alcohólica (EHNA)
Gilead Sciences, Inc. (Nasdaq: GILD) and the Renown Institute for Health Innovation (IHI) today announced a strategic collaboration to collect and analyze genetic and electronic health data that can enhance the understanding of nonalcoholic steatohepatitis (NASH) and potentially inform development of treatment options for the disease. En virtud de los términos del acuerdo de colaboración y licencia, Gilead proporcionará fondos al Renown IHI para secuenciar y analizar el ADN de 15,000 personas que viven con EHNA o con enfermedad de hígado graso no alcohólico (EHGNA), así como de una cohorte de control de 40,000 personas en el estado de Nevada. “Combining the sequencing of protein coding DNA, with extensive electronic health record data will enable a deep analysis of the roles of genetics and environment in NASH incidence and progression,” said John McHutchison, AO, MD, Chief Scientific Officer and Head of Research and Development, Gilead Sciences. “The analysis of these large datasets in collaboration with Renown IHI could help identify genetic variants that impact the risk of developing NASH and thereby advance the discovery and development of new treatments for this disease.” Renown Health es la red de atención médica más completa e integrada de Nevada y mantiene registros médicos electrónicos para 1.02 millones de pacientes registrados. En 2016, Renown Health y el Desert Research Institute crearon el Healthy Nevada Project (HNP), el primer estudio de salud poblacional basado en la comunidad del país. En 2017, el HNP comenzó una sociedad con Helix para aprovechar sus servicios de salud poblacional, la secuenciación Exome+™ y las herramientas de participación del consumidor. The HNP is now an ongoing collaboration between Renown IHI, the Desert Research Institute, a global leader in environmental data and applied research, and Helix, a personal genomics company. HNP combines genetic, environmental, social and clinical data to address individual and community health needs with the goal of improving health across the state and the nation. El HNP actualmente tiene 40,000 participantes. “Combining genetic sequencing with large sets of data can play a critical role in understanding and identifying serious health risks, including diseases like NASH. We are excited to collaborate with Gilead to better understand the condition and its complexities,” said Anthony Slonim, MD., DrPH. “Any genetic variants identified in participants through the collaboration may be shared with the participants for patient care purposes.” About NASH Nonalcoholic steatohepatitis (NASH) is a chronic form of liver disease characterized by excess fat in the liver, inflammation, and liver cell damage. Inflammation and liver cell damage can cause scarring of the liver, or fibrosis, and ultimately lead to cirrhosis or liver cancer. La EHNA es más frecuente en personas con ciertas afecciones, que incluyen obesidad y diabetes tipo 2. There are currently limited approved treatments for patients living with NASH. About Gilead Sciences Gilead Sciences, Inc. is a research-based biopharmaceutical company that discovers, develops and commercializes innovative medicines in areas of unmet medical need. The company strives to transform and simplify care for people with life-threatening illnesses around the world. Gilead opera en más de 35 países alrededor del mundo, con sede central en Foster City, California. Para obtener más información sobre Gilead Sciences, visite el sitio web de la empresa en www.gilead.com. About Renown Health Renown Health is a locally governed and locally owned, not-for-profit integrated healthcare network serving Nevada, Lake Tahoe and northeast California. Renown es uno de los mayores empleadores privados de la región y cuenta con una fuerza laboral de más de 7,000. It comprises three acute care hospitals, a rehabilitation hospital, the area’s most comprehensive medical group and urgent care network, and the region’s largest and only locally owned not-for-profit insurance company, Hometown Health. Renown Health’s commitment has extended beyond traditional health care to include community health and well-being. Renown Health works to improve health care through science, research and genetics; forge community partnerships that improve lives and develop innovative models that are improving health care in Nevada. For more information, visit renown.org. About Helix Helix’s mission is to empower every person to improve their life through DNA. Helix is accelerating the integration of genomic data into clinical care and broadening the impact of large-scale population health programs by providing comprehensive expertise in DNA sequencing, bioinformatics, and individual engagement. Mediante el uso de su ensayo Exome+™ de propiedad exclusiva, un exoma de tipo panel mejorado con más de 300,000 regiones no codificantes informativas, Helix ofrece a los sistemas de salud una solución escalable que permite el descubrimiento de información genética médicamente relevante y que potencialmente puede salvar vidas. Additionally, Helix offers a suite of DNA-powered products for continued individual engagement and discovery. Helix is headquartered in the San Francisco Bay Area and has one of the world’s largest CLIA-certified, CAP-accredited Next Generation Sequencing labs, located in San Diego, California. Obtenga más información en www.helix.com. Helix, the Helix logo, and Exome+ are trademarks of Helix Opco, LLC. Gilead Forward-Looking Statement This press release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that are subject to risks, uncertainties and other factors, including the risk that the parties may not realize the potential benefits of this collaboration, and Gilead may fail to discover, develop and commercialize any product candidates for the treatment of NASH. All statements other than statements of historical fact are statements that could be deemed forward-looking statements. These risks, uncertainties and other factors could cause actual results to differ materially from those referred to in the forward-looking statements. The reader is cautioned not to rely on these forward-looking statements. Estos y otros riesgos se describen en detalle en el Informe Trimestral de Gilead en el Formulario 10-Q para el trimestre que finalizó el 31 de marzo de 2019, presentado ante la Comisión de Bolsa y Valores de los EE. UU. All forward-looking statements are based on information currently available to Gilead, and Gilead assumes no obligation to update any such forward-looking statements. For more information on Gilead Sciences, please visit the company’s website at www.gilead.com, follow Gilead on Twitter (@GileadSciences) or call Gilead Public Affairs at 1-800-GILEAD-5 or 1-650-574-3000. Additional Media Contact: Sung Lee, Investors 650-524-7792 Arran Attridge, Media 650-425-8975
-
Estudio de salud poblacional se extiende a todo el estado y abre la inscripción
The study could expand to a quarter million people, making Nevada the only state in the U.S. to offer such a program. Las Vegas, Nev. (2 de mayo de 2019) – El Healthy Nevada Project, un estudio de salud poblacional basado en la comunidad primero en su clase que combina datos genéticos, clínicos, ambientales y sociales, está ampliando la inscripción a Las Vegas. The Project aspires not only to offer genetic testing to every Nevadan interested in learning more about their health and genetic profile but ultimately, to develop and expand the Project for communities across the United States to drive positive health outcomes nationwide. Se agregarán 25,000 voluntarios del estudio en el sur de Nevada El Healthy Nevada Project anuncia extensión a nivel estatal: abre 25,000 vacantes para pruebas en Las Vegas. Ahora que el sur de Nevada abre sus puertas al estudio, el Healthy Nevada Project ofrecerá pruebas genéticas sin costo mediante una muestra de saliva simple a 25,000 voluntarios del estudio. Study volunteers will take Helix’s clinical-grade DNA saliva test and will receive their ancestry and traits, at no cost, through the My Healthy Nevada Traits app. Participants will then be given a chance to answer a follow-up health survey from Renown Institute for Health Innovation (Renown IHI), and upon survey completion, will be entered to win an iPhone. In addition, study participants can agree to be notified about genetic test results that could impact their health, and which could be used to improve their medical care. This return of clinical results, plus genetic counseling and other genetic services as appropriate, will be provided by Genome Medical, the leading network of clinical genetics specialists. Evolución y expansión continua del Healthy Nevada Project Con más de 35,000 participantes del estudio inscritos en apenas más de dos años, el Healthy Nevada Project se ha convertido en el estudio genético de más rápida inscripción del país. The Project was created by Renown IHI – a collaboration between Reno, Nev.-based not-for-profit health network, Renown Health, and the world leader in environmental data, Desert Research Institute (DRI). Leveraging Renown’s forward-thinking approach to community health care and DRI’s data analytics and environmental expertise, Renown IHI has grown its capabilities to lead a larger, more complex research study of significance that will analyze and model public health risks in Nevada and serve as a national model for future population health studies working to improve overall health through clinical care integration. En el lanzamiento piloto del proyecto el 2016 de septiembre, se inscribieron más de 10,000 miembros de la comunidad para realizarse pruebas de ADN en solo 48 horas. En marzo de 2018, en la fase dos, se les ofreció a los residentes del norte de Nevada secuenciación genómica completa mediante una simple prueba de saliva de Helix. En octubre de 2018, el proyecto anunció la devolución de los resultados clínicos a los participantes del estudio. Allí se notificaba el riesgo que tenían de padecer afecciones de Nivel 1 de los CDC, que incluyen hipercolesterolemia familiar, BRCA1 y BRCA2 positivo, y síndrome de Lynch, un precursor del cáncer de colon. These conditions affect more than one percent of the population and are inherited so they impact family members as well. Ahora, el proyecto anunció su próxima fase: la extensión de la inscripción a 25,000 personas del sur de Nevada. Serving as a National Model This expansion to Las Vegas truly makes this the “Healthy Nevada Project” with a statewide impact making Nevada the only state in the U.S. to offer such a program. “Nevada was ripe to advance population health goals because, sadly, our state ranks near the bottom in health outcomes. The Healthy Nevada Project is working to change that,” said Anthony Slonim, M.D., DrPH, FACHE, president and CEO of Renown Health and president of Renown IHI. “Nuestros investigadores están trabajando en una serie de programas clínicos y estudios científicos para determinar por qué en el condado de Washoe, el condado en el que se encuentra Renown Health, las tasas de mortalidad etarias para enfermedad cardíaca, cáncer y enfermedad crónica de las vías respiratorias inferiores de Nevada son un 33 por ciento más altas que la tasa nacional. Imagine if we can gather more data like this on a national scale and use it to change the future of health and health care? That is what the Healthy USA Project is looking to do in the years to come.” “The Healthy Nevada Project is committed to providing study participants clinically actionable data that will help improve their health,” said Joseph Grzymski, Ph.D., associate research professor at DRI, principal investigator of the Healthy Nevada Project and chief scientific officer for Renown Health. “We are providing this information at the individual level so study volunteers can make lifesaving changes to reduce their risk. We’re also doing it on the community level to develop leading-edge research on health determinants for entire neighborhoods, states and eventually, the country.” Expanding to Become the Healthy USA Project The accelerated speed of the Project is made possible thanks to the ever-decreasing cost of sequencing. Hoy en día, Helix puede secuenciar un exoma completo, lo que permite informar sobre la mayoría de los conocimientos genómicos prácticos, por una fracción de lo que habría costado hace solo 10 años. Additionally, advances in digital health mean Helix and Project researchers can capture unprecedented amounts of health data digitally, making significant contributions to advancing precision health. The partnership has managed to remove the traditional barriers of population health studies, including the difficulty in recruiting participants, establishing quality high through put lab systems, and scaling interpretation and return of results. This development will be key as other health systems around the country join the Project. “We are thrilled to see the constant, fast-paced evolution of this Project with Renown IHI,” said Justin Kao, CoFounder of Helix. “In less than a year, we have sequenced the DNA of thousands of study participants and are now preparing to offer this incredible study in other states. Combining environmental, clinical, social and genetic data allows us to discover risk factors within communities and help people take action to live longer, healthier lives. That’s what makes the next step of the Healthy USA Project so exciting for all of us. “Los detalles completos sobre la evolución del estudio y de la inscripción en el sur de Nevada se darán a conocer en un evento de prensa en Las Vegas el martes 7 de mayo a las 3 p.m. Renown Institute for Health Innovation es una colaboración entre Renown Health, una red de atención médica integrada de administración y propiedad local y sin fines de lucro que brinda servicios a Nevada, Lake Tahoe y el noreste de California, y el Desert Research Institute, líder mundial reconocido en la investigación de los efectos del cambio ambiental natural e inducido por el humano y en la promoción de tecnologías para la evaluación de un planeta cambiante. Renown IHI research teams are focused on integrating personal healthcare and environmental data with socioeconomic determinants to help Nevada address some of its most complex environmental health problems; while simultaneously expanding the state’s access to leading-edge clinical trials and fostering new connections with biotechnology and pharmaceutical companies. Obtenga más información en https://healthynv.org/. Helix es una empresa de genómica con una misión simple pero potente: to empower every person to improve their life through DNA. Our affordable, turnkey population health solution enables institutions to quickly scale projects that engage communities and accelerate research and discovery, ultimately allowing every person to benefit from the power of genomics. We've also created the first marketplace for DNA-powered products where people can explore diverse and uniquely personalized products developed by high-quality partners, providing powerful tools to increase engagement and speed the pace of population-scale genomics. Helix is headquartered in the San Francisco Bay Area, has an office in Denver, Colorado and operates a CLIA-certified and CAP-accredited next-generation sequencing lab in San Diego powered by Illumina (NASDAQ: ILMN) NGS technology. Helix fue creada en 2015. Obtenga más información en www.helix.com. Helix, el logotipo de Helix y Exome+ son marcas comerciales de Helix Opco, LLC. All other trademarks referenced herein are the property of their respective owners. Additional Media Contacts: Tracy Bower DRI 702-862-5404 media@dri.edu Eric Schubert Seismic for Helix 415-939-4366 press@helix.com
Leer más About Estudio de salud poblacional se extiende a todo el estado y abre la inscripción
-
Presentación de Dana, el nuevo quiosco holográfico tamaño real del Renown Institute for Health Innovations
Presented by the Desert Research Institute and Renown Health, DANA will help people learn more about the Healthy Nevada Project® and their own unique, genetic health traits What is the Healthy Nevada Project®? What are the benefits of joining this research study? How can I find out if I carry genes for health risks like heart disease, hereditary breast and ovarian cancer syndrome and Lynch syndrome? What if a holographic avatar, powered by artificial intelligence (AI), could answer all these questions and more? DANA has all the answers, she is a virtual assistant with “DNA” in her name, presented by the Renown Institute for Health Innovation (Renown IHI), a collaboration between Renown Health and the Desert Research Institute (DRI). This life-sized, holographic avatar will greet individuals outside Renown Regional Medical Center’s Sierra Café, and talk to them about the Healthy Nevada Project, the world’s largest community-based genetic population health study. Los representantes de medios de comunicación están invitados a reunirse con DANA esta mañana, desde las 10 a.m. hasta el mediodía. Responda a este correo electrónico o llame al (775) 691-7308 para coordinar una reunión. “Unfortunately, Nevada ranks among the lowest in the nation for health outcomes—and we are working to change that,” said Anthony Slonim, M.D., D.Ph., president and CEO of Renown Health and president of Renown Institute for Health Innovation. “Through the Healthy Nevada Project® , our goal is to offer genetic testing to every Nevadan interested in learning more about their health and genetic profile. Thanks to this advanced technology, DANA will offer people a personalized explanation of the Project, and help them take the next step to better understand their health, and their health risks, so they can modify their behavior and ultimately, live a healthier, happier life.” Con más de 51,000 participantes del estudio inscritos hasta la fecha, el Healthy Nevada Project® se considera el estudio genético de más rápida inscripción del país. The Project is also the first of its kind to return clinical results to study volunteers, which means participants can learn their genetic risks tied to heart disease and certain cancers, as well as lifestyle changes that could potentially help reduce their risk and prevent disease. Furthermore, participants can choose to share their information with their medical provider to improve and enhance their medical care. “We are always happy to engage with our study participants and look forward to having them meet and engage with DANA,” said Joseph Grzymski, Ph.D., research professor at DRI, principal investigator of the Healthy Nevada Project® and chief scientific officer for Renown Health. “At a time of physical distancing and limiting human contact where possible, using tools like an avatar and AI are important for communicating, whether it be for genetics, vaccinations or other important health information.” Visitors can interact with DANA through a touch screen (cleaned and sanitized after every encounter) to learn more about the study, enter their contact information and schedule an appointment to join the free genetics study or receive more information about their test results. Kiosk visitors are asked to maintain physical distance guidelines and use the hand sanitizer and Sani Wipes available next to the kiosk. Renown Institute for Health Innovation is a collaboration between Renown Health - a locally governed and locally owned, not-for-profit integrated healthcare network serving Nevada, Lake Tahoe and northeast California; and the Desert Research Institute - a recognized world leader in investigating the effects of natural and human-induced environmental change and advancing technologies aimed at assessing a changing planet. Renown IHI research teams are focused on integrating personal healthcare and environmental data with socioeconomic determinants to help Nevada address some of its most complex environmental health problems; while simultaneously expanding the state’s access to leading-edge clinical trials and fostering new connections with biotechnology and pharmaceutical companies. Learn more at healthynv.org.
-
Population Genetic Screening Show to Efficiently Identify Increased Risk for Inherited Disease
El enfoque basado en la comunidad del Healthy Nevada Project revela que hasta un 90 % de los riesgos de padecer afecciones genéticas de Nivel 1 de los CDC no se detecta cuando se usan pautas de atención clínica. In a new study published today in the journal Nature Medicine, researchers behind the Healthy Nevada Project® suggest that community-based genetic screening has the potential to efficiently identify individuals who may be at increased risk for three common inherited genetic conditions known to cause several forms of cancer and increased risk for heart disease or stroke. En 2018, el Healthy Nevada Project® (el mayor estudio de salud poblacional basado en la comunidad que combina datos genéticos, clínicos, ambientales y sociales) comenzó a notificarles a los participantes del estudio que habían dado su consentimiento sobre ciertas variantes genéticas que los predisponían a padecer las afecciones genéticas de Nivel 1 de los Centros para el Control y la Prevención de Enfermedades (Centers for Disease Control and Prevention, CDC). The study focused on identifying carriers of these conditions, which include Hereditary Breast and Ovarian Cancer, Lynch Syndrome, and Familial Hypercholesterolemia, because they are the most common conditions and early detection and treatment could significantly lower morbidity and mortality. Los resultados iniciales de casi 27,000 participantes del estudio mostraron que el 90 % de los portadores de las afecciones genéticas de Nivel 1 de los CDC no había sido previamente identificado en un entorno clínico. The authors conclude that population genetic screening would identify at-risk carriers not identified during routine care. “Our first goal was to deliver actionable health data back to the participants of the study and understand whether or not broad population screening of CDC Tier 1 genomic conditions was a practical tool to identify at-risk individuals,” explained Joseph Grzymski, Ph.D., the principal investigator of the Healthy Nevada Project®, a research professor at the Desert Research Institute (DRI), chief scientific officer for Renown Health and lead author of the study. “Now, two years into doing that, it is clear that the clinical guidelines for detecting risk in individuals are too narrow and miss too many at risk individuals.” Del grupo de 26,906 participantes del Healthy Nevada Project® que el equipo de investigación de Grzymski estudió, 358 (el 1.33 %) eran portadores de afecciones de Nivel 1 de los CDC. Sin embargo, solo el 25 % de esas personas cumplían las pautas clínicas para realizarse exámenes de detección genética. Además, más del 20 % de los portadores ya tenía un diagnóstico de enfermedad relacionada con la afección genética subyacente. “We’re at a point now where it’s possible to do clinical-grade genetic screening at population-scale,” added James Lu, M.D. Ph.D., co-founder and chief scientific officer of Helix and senior co-author of the study. “What this study demonstrates is the potential impact of doing so. By making genetic screening available more broadly, we can help the millions of Americans who are unaware that they are living at increased risk for highly actionable, genetic conditions take action.” En particular, el estudio descubrió que de los 273 participantes que eran portadores de las afecciones genéticas de Nivel 1 de los CDC y que tenían información de registros clínicos, solo 22 personas mostraron sospechas previas de tener estas afecciones genéticas subyacentes. “For the first time, we are providing information at the individual level so study participants can make lifesaving changes to reduce their risk based on their genetics,” said Anthony Slonim, M.D., Dr.PH., FACHE, president and CEO of Renown Health and co-director of the Project® study. “We’re conducting research on the community level to develop leading-edge research on health determinants for entire neighborhoods, states and eventually, the country. Returning these results allows us to understand the prevalence of genetically programmed diseases and illnesses that we have here in Nevada and ensure we are providing the best prevention and care plans. For the individual, the return of results can be life changing.” According to the CDC, early detection and intervention of the Tier 1 genetic conditions could have a meaningful potential for clinical action ability and a positive impact on public health. El Healthy Nevada Project®, que se lanzó en 2016, ofrece pruebas genéticas gratuitas a todos los residentes de Nevada, mayores de 18 años, interesados en obtener más información sobre su salud y perfil genético. Con más de 50,000 participantes del estudio inscritos en cuatro años, el Healthy Nevada Project® se ha convertido en el estudio genético de más rápida inscripción del mundo. For more about the Healthy Nevada Project® please visit healthynv.org Renown Institute for Health Innovation is a collaboration between Renown Health – a locally governed and locally owned, not-for-profit integrated healthcare network serving Nevada, Lake Tahoe and northeast California; and the Desert Research Institute – a recognized world leader in investigating the effects of natural and human induced environmental change and advancing technologies aimed at assessing a changing planet. Renown IHI research teams are focused on integrating personal healthcare and environmental data with socioeconomic determinants to help Nevada address some of its most complex environmental health problems; while simultaneously expanding the state’s access to leading-edge clinical trials and fostering new connections with biotechnology and pharmaceutical companies. Learn more at Healthynv.org. Helix is the leading population genomics company operating at the intersection of clinical care, research, and genomics. Its end-to-end platform enables health systems, life sciences companies, and payers to advance genomic research and accelerate the integration of genomic data into clinical care. Powered by one of the world's largest CLIA / CAP next-generation sequencing labs and its proprietary Exome+Ⓡ assay, Helix supports all aspects of population genomics including recruitment and engagement, clinically actionable disease screening, return of results, and basic and translational research. In response to the COVID-19 public health crisis, Helix has launched a sensitive and scalable end-to-end COVID-19 test system to meet the needs of health systems, employers, governments, and other organizations across the country. Obtenga más información en www.helix.com.
-
Newly Expanded Clinical Research Center at UNR Med Fosters Collaboration and Research with Renown Health
Renown Health and the University of Nevada, Reno School of Medicine (UNR Med) are proud to announce a newly integrated and expanded research space called the Clinical Research Center (CRC). This space offers a dynamic physical location on the University of Nevada, Reno campus that supports the UNR Med and Renown Health research enterprise. "The partnership between Renown Health and UNR Med truly knows no bounds, and this Clinical Research Center is an incredible example of that endless possibility,” said Thomas Graf, MD, interim CEO of Renown Health. “This new space will only continue to expand our community’s access to clinical research as part of patient care while providing the necessary resources to engage our students and support a healthy Nevada.” This space’s capabilities include experienced staff with knowledge and skills in operationalizing FDA and non-FDA regulated clinical and translational research studies in a centralized 5,470-square foot research clinic housed in the Center for Molecular Medicine (CMM) at the University. This CRC space provides resources including: A centralized location next to the laboratory space that allows for strategic interdisciplinary collaboration between clinicians and basic scientists. Eleven private outpatient rooms for research clinic visits. Two blood draw stations. Physician consultation areas. Conference room for trial monitoring and consulting. Secure Investigational Product storage and preparation. Sample processing and storage, including countertop refrigerated centrifuges, 4°, -20° and -80° C refrigerators and freezers. Operations around clinical research are becoming more complex so growing clinical research in our community will require expertise and dedicated space where clinical research can be conducted in a learning environment first,” said Danielle Eaton, Director of Clinical Research with UNR Med and Renown Health. “This Clinical Research Center provides such space and experienced staff where these research studies can be successfully completed. The CRC provides a training environment for students, residents, faculty and clinical research professional work-force that will be needed to bring cutting edge diagnostics, therapeutics and preventatives to our community.” Meet the Team: Danielle Eaton, UNR Med Director of Clinical Research Kristen Gurnea, Renown Health Manager of Clinical Research Amber Emerson, UNR Med Project Manager Valerie Smith, UNR Med Center Administrative Manager Annie Beach-Hills, Gina Castro, Michelle Mejia and Amil Trujillo-King, UNR Med Study Coordinators Dr. John Westhoff, UNR Med Chair of Internal Medicine, Emergency Medicine physician Dr. Sean Kandel, UNR Med Associate Program Director for Resident Research, Associate Professor of Internal Medicine Dr. Amneet Rai, UNR Med Clinical research pharmacist Dr. Kellie Watkins, UNR Med Clinical Epidemiologist/Data Manager/Statistician As part of the affiliation between UNR Med and Renown Health, the Clinical Research Office is part of an integrated Office of Clinical Research, which allows both entities to collaborate on shared research program objectives. This effort allows colleagues to partner on clinical research, and to leverage bench-to-bedside research and delivery of leading-edge healthcare to northern Nevadans. Acerca de Renown Health Renown Health es la red de atención médica integrada de administración local y sin fines de lucro más grande de la región, que presta servicios a Nevada, Lake Tahoe y el noreste de California. Con una fuerza laboral diversa de más de 7,000 empleados, Renown ha fomentado una cultura de excelencia, determinación e innovación de larga data. La organización se compone de un centro de urgencias, dos hospitales de cuidados agudos, un hospital infantil, un hospital de rehabilitación, un grupo médico y una red de atención de urgencias y Hometown Health, la compañía de seguros sin fines de lucro más grande de la región y de propiedad local, Hometown Health. Actualmente, Renown está inscribiendo participantes en el estudio genético de salud poblacional basado en la comunidad más grande del mundo, el Healthy Nevada Project®. About UNR Med The University of Nevada, Reno School of Medicine (UNR Med), Nevada’s first public medical school, is a community-based, research-intensive medical school with a statewide vision for a healthy Nevada. Desde 1969, UNR Med ha capacitado a más de 3,900 estudiantes, residentes y becarios. UNR Med continues to improve the health and well-being of all Nevadans and their communities through excellence in student education, postgraduate training and clinical care, research with local, national and global impact and a culture of diversity and inclusion. For more information, visit med.unr.edu.
-
Estudios clínicos de neurociencias
Neurosciences Clinical Trial Opportunities
-
Sobreviviente del cáncer de ovario cuenta cómo decidió probar con ensayos clínicos
While there used to be three basic treatment options for cancer -- surgery, radiation and chemotherapy, or a combination of the three -- there's a fourth option: clinical trials. Here, a Renown patient shares her successful battle with ovarian cancer, aided by a clinical trial. Shari Flamm's battle with ovarian cancer began in 2011. She was experiencing prolonged bleeding, irregular thyroid levels and anemia and was scheduled to undergo a hysterectomy. Before the surgery, her gynecologist ran routine tests to check for cancer as a precautionary measure. All tests were negative for cancer, expect her CA 125 test. A CA 125 test measures the amount of the protein CA 125 (cancer antigen 125) in the blood. In some cases, a CA 125 test may be used to look for early signs of ovarian cancer in women with a very high risk of the disease. In most laboratories, the normal level is 0 to 35 units/ml. Flamm's CA 125 level was 121. As Flamm can attest, early diagnosis played a key role in her battle with ovarian cancer. September is Gynecologic Cancer and Ovarian Cancer Awareness Month – an important time to learn the signs, symptoms and risk factors of this type of cancer so your doctor can diagnosis the disease as early as possible. Ovarian Cancer: Round One Despite the elevated CA 125 results, her doctor recommended they move forward with the hysterectomy. But when surgery began, doctors discovered a mass. She had stage 4 cancer. The procedure was halted, the mass was biopsied and she was immediately seen by Dr. Peter Lim of the The Center of Hope. Following diagnosis, Flamm underwent surgery with Dr. Lim to remove the cancer, which had spread to part of diaphragm, spleen, colon and other organs. Three months after surgery, Flamm had recovered enough to start six rounds of chemotherapy in her hometown of Carson City. She continued working at a doctor's office during her treatment, and was grateful for Dr. Lim’s ability to co-manage her care so she could stay close to work and family. “To me, chemo was the scariest part because I didn’t like feeling sick,” Flamm says. Thankfully, her body responded well to the treatments and she was back to the things she loved. “I stated working out at the gym, even if it was only for 10 minutes,” she says. She also stayed positive by spending time with her grandchildren, attending a San Jose Sharks hockey game, going for walks and enjoying concerts. Ovarian Cancer: Round Two In November 2014, Flamm had a cancer check-up. That’s when doctors discovered three cancerous tumors. For this round, Flamm choose another treatment option -- clinical trials at Renown Institute for Cancer. Clinical trials are the studies that test whether drugs work, and inform doctors' decisions about how to treat their patients. Flamm participated in a clinical trial that featured oral-targeted therapy stronger than IV chemotherapy. The hope was for the drug to shrink her tumors, however the result was stabilization -- meaning the lumps weren’t growing or spreading. The best part of the clinical trial, Flamm says, was the constant monitoring. Between the CT scans every six weeks, a heart scan every three months and monthly doctor visits, she was confident that if the cancer started growing or spreading, her healthcare team would catch it right away. For Flamm, the benefits of the clinical trial included less hair loss, less fatigue and more time to focus on what’s important in her life -- her family. “I decided I wasn’t going to be that sick grandma on the couch with cancer,” Flamm says. After taking the oral medication for one year, Flamm developed a rash and discontinued treatment due to discomfort. Clinical Trials, Setbacks and Survival In June 2016, two of the three tumors began to grow and had to be surgically removed. Despite the setback, Flamm was determined to maintain a positive outlook. "You have to stay positive because cancer feeds off anger, depression and stress," Flamm says. Flamm was released to go home with clear margins, meaning the tumors were removed and are surrounded by a rim of normal tissue that does not have cancerous cells. Flamm says her outlook on life has changed drastically since her first cancer diagnosis. “Your whole mentality changes when cancer disturbs your life," Flann says. "The things that weren’t important, are now ever so important. I’m a lot calmer now,” Flamm says.
Read More About Ovarian Cancer Survivor Shares Decision to Try Clinical Trial
-
¿Qué significa participar en un estudio clínico?
Participating in a clinical trial is voluntary and a personal choice. Clinical trials are research studies that involve people and are an important part of patient care. What is a clinical trial? Clinical trials are research studies that involve people, and they are an important part of patient care. There are several different types of clinical trials; some are designed to understand trends in a disease or identify better ways to diagnose a condition, while others determine if a new treatment is safe and works when treating, improving or preventing a health condition. There are over 400,000 clinical trials currently being conducted in the United States, and even more across the world. This includes health conditions such as heart failure, cancer, Parkinson’s Disease, respiratory conditions like COPD, common infections, cystic fibrosis, and many more. Clinical trials lead the healthcare industry to new discoveries that contribute to reliable and exact care, improving healthcare quality and saving lives. Clinical trials are conducted by a team of researchers, including doctors, pharmacists and clinical research coordinators. These research teams are highly skilled in their specialty areas, often providing traditional patient care and seeing research patients in the same day. These teams are responsible for making sure the clinical trial is completed correctly, and their patients are their top priority. Why should I consider participating in a clinical trial? Participating in a clinical trial is voluntary and a personal choice. There are many reasons why patients decide to get involved in clinical research. While many clinical trials are designed for patients who have a certain health condition, many studies also ask healthy volunteers to contribute in order to compare health outcomes. Clinical trials are also for patients at all different stages of their diagnosis. Depending on the specific study, the patient may receive access to a new cutting-edge treatment before it is widely available. When patients join a clinical trial, the research team becomes a health partner dedicated to their health and well-being. When patients join a clinical trial, they make an informed decision in their healthcare by weighing all available options in addition to routine treatments. Research participants know that they are contributing meaningfully and helping other patients like them. Where can I find more information about clinical trials at Renown Health? Renown Health’s mission is to make a genuine difference in the health and well-being of the communities we serve. Renown’s clinical trial portfolio offers leading care options to patients in northern Nevada, close to home, in a variety of specialties. Contact the Renown Clinical Research Office for more information on clinical trials available to you!
Read More About What Does It Mean to Participate in a Clinical Trial?
-
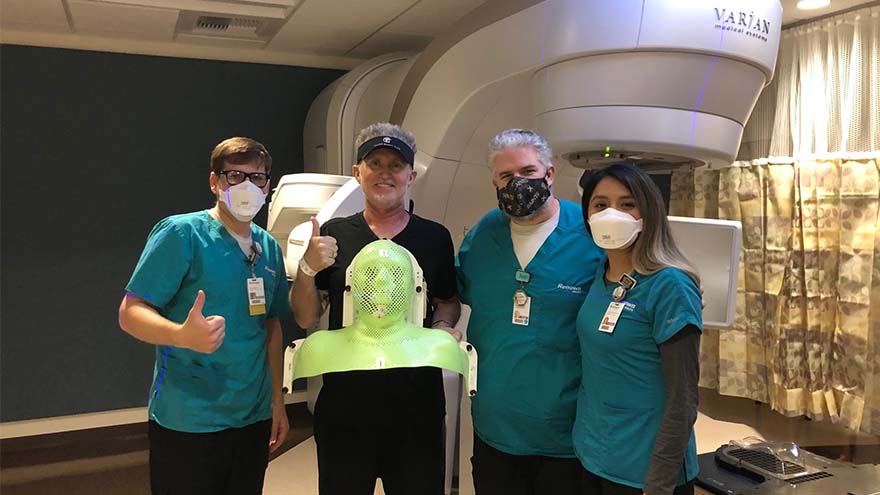
Un diagnóstico de cáncer y una mudanza a Reno
Michael Millman was all set to move to Reno from the Bay Area when he noticed a pimple-like growth on his forehead, and he decided to get biopsied "just in case." It was July 2020, less than six months into the COVID-19 pandemic, when Michael got the call that the biopsy came back cancerous. He was in shock. Still living in the Bay Area at the time, he immediately scheduled to have the basal cell carcinoma removed in August. After the removal, he thought he was in the clear, but a few months later, Michael noticed that his lymph nodes felt weird, and he even cut himself shaving because of some persistent swelling in the area. Given his recent history of skin cancer, Michael immediately scheduled an appointment with a specialist in the Bay Area. "I met with an ear, nose and throat doctor who suggested a fine needle biopsy of my lymph nodes, tongue and an MRI, both with and without contrast," Michael said. "I remember feeling dreadful and that I couldn't believe this was happening yet again." A Hard Decision Michael's squamous cell carcinoma, determined by the pathology report to be significantly influenced by the HPV virus, had metastasized to his lymph nodes on both sides of his neck, and his doctor said it could be stage four cancer. He remembers feeling like he was in quicksand, unsure if he should follow through with his move to Reno, or stay in the Bay Area for treatment. By now, it was early December 2020, and hospitals in the Bay Area and across the world were at limited capacity due to COVID-19. But, in what Michael describes as a positive twist of fate, the San Francisco ear, nose and throat provider he had seen about his biopsy results mentioned that he knew many providers in the oncology department at Renown, including Abhinand Peddada, MD. The San Francisco provider called Dr. Peddada's office with a referral, and Michael even remembers that Renown called him to hear more about his diagnosis before he even got the chance to call them "To be honest, I was feeling shut out in the Bay Area, and Dr. Peddada said he could help me expedite the treatment process," Michael said. "I finally felt a sense of relief." And so began Michael's 7-week chemoradiation cancer treatment program at Renown.