Buscar
-
¿Qué significa participar en un estudio clínico?
Participating in a clinical trial is voluntary and a personal choice. Clinical trials are research studies that involve people and are an important part of patient care. What is a clinical trial? Clinical trials are research studies that involve people, and they are an important part of patient care. There are several different types of clinical trials; some are designed to understand trends in a disease or identify better ways to diagnose a condition, while others determine if a new treatment is safe and works when treating, improving or preventing a health condition. There are over 400,000 clinical trials currently being conducted in the United States, and even more across the world. This includes health conditions such as heart failure, cancer, Parkinson’s Disease, respiratory conditions like COPD, common infections, cystic fibrosis, and many more. Clinical trials lead the healthcare industry to new discoveries that contribute to reliable and exact care, improving healthcare quality and saving lives. Clinical trials are conducted by a team of researchers, including doctors, pharmacists and clinical research coordinators. These research teams are highly skilled in their specialty areas, often providing traditional patient care and seeing research patients in the same day. These teams are responsible for making sure the clinical trial is completed correctly, and their patients are their top priority. Why should I consider participating in a clinical trial? Participating in a clinical trial is voluntary and a personal choice. There are many reasons why patients decide to get involved in clinical research. While many clinical trials are designed for patients who have a certain health condition, many studies also ask healthy volunteers to contribute in order to compare health outcomes. Clinical trials are also for patients at all different stages of their diagnosis. Depending on the specific study, the patient may receive access to a new cutting-edge treatment before it is widely available. When patients join a clinical trial, the research team becomes a health partner dedicated to their health and well-being. When patients join a clinical trial, they make an informed decision in their healthcare by weighing all available options in addition to routine treatments. Research participants know that they are contributing meaningfully and helping other patients like them. Where can I find more information about clinical trials at Renown Health? Renown Health’s mission is to make a genuine difference in the health and well-being of the communities we serve. Renown’s clinical trial portfolio offers leading care options to patients in northern Nevada, close to home, in a variety of specialties. Contact the Renown Clinical Research Office for more information on clinical trials available to you!
Read More About What Does It Mean to Participate in a Clinical Trial?
-
Top 5 Misconceptions About Clinical Trials
There are many misconceptions about clinical research, so we have unpacked a few common myths we hear to help you make an informed decision in your healthcare. Misconception #1: If I join a clinical trial, I’ll just be a guinea pig. Quite the opposite is true! Through honest and respectful conversation, we ensure all participants are informed of the benefits and risks associated with the clinical trial during the informed consent process. Being in a clinical trial is voluntary, and we respect our patients’ decision to join or decline to participate in the clinical trial. You can always change your mind at any time as well. When patients join a clinical trial, they receive an additional team of healthcare professionals, including additional physicians, pharmacists and research coordinators, dedicated to their safety and well-being. This means that clinical trial participants often receive more support than they would in the standard treatment setting. Misconception #2: Clinical trials are too dangerous because they use new treatments that haven’t been tested. We recognize that there are different levels of risk associated with participating in a clinical trial depending on the type of study. However, new treatments are only reviewed through clinical trials after they have gone through extensive testing. New treatments that do not show promising results for safety and potential benefit during laboratory testing do not receive approval to begin clinical trials. Your research team reviews any expected benefits and risks identified from previous studies during the informed consent process, as well as any updates that occur throughout the duration of the clinical trial. The research team stays in close contact with you during the entire process, documenting and treating any side effects that you experience for both your safety and the safety of participants like you. Misconception #3: I don't want to join a trial because I could be wasting my time receiving a placebo. A placebo is a substance that has no therapeutic effect, sometimes called a “sugar pill.” Participants who receive a placebo during a clinical trial are very important, helping researchers definitively determine the specific good and bad effects of the new medication. Many clinical trials that involve a placebo also offer what is called an open label extension or cross-over study. Cross-over studies ensure that anyone taking the placebo can begin receiving the new medication, often for several years. Cross-over studies help clinician researchers understand the long-term effects of a medication while also giving patients free access to novel care for several months and even years.
-
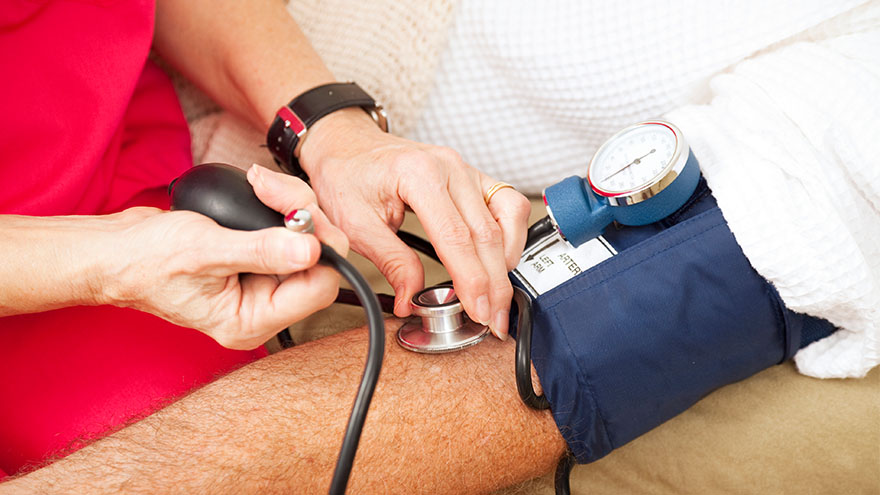
Managing Your Uncontrolled High Blood Pressure
Renown Health, the region's leading cardiology care provider, is offering a clinical trial for eligible patients struggling to control their high blood pressure. Nearly half of adults (119.9 million) in the United States have hypertension, or blood pressure that is higher than normal. Hypertension can put you at risk of other life-threatening disease, such as a heart attack or stroke. There are methods that cardiologists use to manage high blood pressure, but only 1 in 4 adults with hypertension (27.0 million) have their blood pressure under control.* Some patients with high blood pressure experience resistant hypertension, which does not respond well to multiple antihypertensive medications given at the same time. This means that there are many important opportunities for healthcare professionals to explore new ways to treat hypertension. At Renown Health, we lead the region in cardiology care with our technological expertise and patient-centered approach. That is why our cardiology team is partnering with the Renown Research Office to offer the RADIANCE Continued Access Protocol (RADIENCE CAP) clinical trial to eligible patients. RADIANCE CAP is a non-randomized study designed to allow for continued access to ultrasound renal denervation therapy via the Paradise System, and to allow for the on-going collection of safety and effectiveness data in patients with uncontrolled hypertension despite the prescription of antihypertensive medications. The body’s complex communication system between the brain, heart and kidneys can sometimes become overactive, increasing your blood pressure through messages in the nervous system. Renal denervation is a minimally-invasive procedure which reduces activity from the nerves in your kidneys to lower blood pressure. This is the third in a series of renal denervation clinical trials Renown Health has offered to patients with resistant hypertension over the last several years, with over 40 local participants. “All the participants that I have had the pleasure to work with on these studies are very excited and grateful to have this option for helping control their blood pressure” states Lisa English, Lead Clinical Research Coordinator for Cardiology studies at Renown Health. “I love getting to know each one of them and helping on their healthcare journey. We have an amazing team of providers and staff at Renown that go out of their way to make patients experiences positive and the studies successful.” Dr. Michael Bloch, Cardiologist and Principal Investigator for the RADIANCE CAP study at Renown Health’s Institute for Heart and Vascular Health adds, “Despite lifestyle modifications like diet and exercise and the widespread availability of effective and well-tolerated medications, approximately 50% of all people with hypertension have inadequate blood pressure control putting them at risk for stroke, heart failure and kidney disease. As a one-time durable procedure, renal denervation with the Paradise endovascular system from ReCor Medical, Inc. may help millions of patients improve their blood pressure control without necessarily needing to increase their medications.” Our teams of expert providers and researchers are here to support you on your healthcare journey. Talk to your provider about the RADIANCE CAP clinical trial at your next appointment to see if participation may be right for you.
Read More About Managing Your Uncontrolled High Blood Pressure
-
6 Healthcare Action Items for the LGBTQIA+ Community
Every patient, regardless of how they may identify, greatly benefits from preventive healthcare and early detection. Members of the LGBTQIA+ community face unique considerations when it comes to their health, and a proactive approach to preventive screenings and vaccines is important in order to address their individual health needs. Dr. Karen Thiele, Family Medicine Physician with University Health and Assistant Professor of Family and Community Medicine at the University of Nevada, Reno School of Medicine, breaks down key steps that LGBTQIA+ patients should take to safeguard their health. PrEP and PEP Pre-exposure prophylaxis (PrEP) is a strategy to prevent human immunodeficiency virus (HIV) infection. It is an important measure for those who are HIV-negative but may be at risk of contracting it. The highest risk sexual practice is receptive anal intercourse, due to the relative fragility of rectal tissue. This medication can stop HIV from spreading in the body and help patients maintain their HIV-negative status. PrEP is available in both pill form, which is taken every day, and injection form, of which the first two injections are initiated one month after another while all other injections are initiated every two months. Post-exposure prophylaxis (PEP) is an antiretroviral drug regimen taken after potential HIV exposure to prevent an HIV-negative individual from converting to HIV-positive status. PEP is only for emergency situations and must be started within 72 hours of exposure – sooner is always better than later – and must be taken for 28 days. PrEP and PEP are available in many ways, including visiting your primary care provider (PCP) or an urgent care location. HPV Immunization All genders and identities can protect themselves against human papillomavirus (HPV), a sexually transmitted infection (STI) that can lead to the risk of cervical, mouth, head, neck, throat, anal, vaginal, penile and vulvar cancers. HPV is so common that nearly all sexually active people, regardless of sexual orientation and practices, will be exposed at some point in their lifetime. The HPV vaccine (common brands include Gardasil and Cervarix) is a safe and effective method to prevent HPV, according to the Centers for Disease Control and Prevention (CDC). This vaccine protects against infections that can lead to HPV-related cancers and precancers, as well as genital warts. While patients should start receiving the vaccine at 9 years old years old, unvaccinated adults up to the age of 45 can also receive the vaccine through their PCP – better late than never! STI Testing Sexually-transmitted infections form from bacteria, viruses or parasites that can be transmitted by person-to-person sexual contact through semen, vaginal, blood and other bodily fluids. According to the U.S. Department of Health and Human Services, there are more than 20 million estimated new STI cases across the nation each year. Luckily, most STIs are preventable. Annual STI testing for HIV, gonorrhea, chlamydia and syphilis is important to stay on top of your sexual health. Because these STIs may sometimes have no symptoms, screening is recommended regularly and with any change in sexual partners. Depending on the specific condition, tests for these infections include urine, swab and blood tests. Speak with your primary care provider on a screening schedule that works best for you. Prostate Exams Prostate exams look for early signs of prostate cancer in patients who still have a prostate. The CDC recommends those who are at least 55 years old get regular prostate screenings; however, for patients with a family history of prostate cancer, screenings may be recommended as early as 45 years old. These exams are done via two common methods – a prostate specific antigen (PSA) blood test and a digital rectal examination (DRE). Your provider can help you determine your risk and when you should start getting screened. Pap Tests and Pelvic Exams Patients of all genders who have a cervix, uterus, vagina and/or ovaries will benefit from regular pelvic exams and Pap screenings. A pelvic exam consists of a provider looking inside the vagina and at the cervix for anything unusual. A Pap test, also known as a Pap smear, involves your provider using a small, soft swab to collect cervical cells to check for early signs of cancer. Generally speaking, people with these organs should have a Pap test every three years starting at age 21 through the age of 30. After age 30, patients should receive a Pap test with HPV co-testing every five years until age 65. These recommendations are changing based on new research, so it is important to have a conversation with your PCP about the current guidelines so you can make an informed choice about what schedule you should follow. A gynecologist or your primary care provider can counsel you and perform these screenings. Mammograms and Breast Exams People with breast tissue, especially dense breast tissue, are at risk for breast cancer, and regular breast screenings are your best line of defense. At-home breast self-exams are the first step – you will want to check your breasts for any lumps, changes, fluid leaks, irregular tissue thickening or anything else that feels unusual. The Breast Cancer Risk Assessment tool, provided by the National Cancer Institute, is a good place to start to identify your risk. Talk with your primary care provider about the risks and benefits of starting screening at age 40 so you can make an informed decision about when to start. If you have any family history of breast or ovarian cancer, your PCP will offer you genetic testing for BRCA 1 and 2 mutations. Nevadans over the age of 18 can also get BRCA genetic test for free by enrolling in the Healthy Nevada Project. Mammograms are important screening tools, but for a significant portion of people with breast tissue, density of the breast tissue may make mammograms less helpful in detecting cancer. Your primary care provider can help you decide what additional imaging (such as breast ultrasound) might be best for you.
Read More About 6 Healthcare Action Items for the LGBTQIA+ Community
-
Multiple Sclerosis Research Opportunities in Northern Nevada
There are nearly 1 million adults living with MS in the United States alone. For comparison, that is roughly the entire population of the Reno/Sparks and Las Vegas areas combined. MS is a neurological autoimmune condition which means that the immune system of patients with MS attacks the body’s myelin, a protective substance that covers your nerves. When this happens, the unprotected nerves can be damaged. Patients with MS may experience many different symptoms ranging from mild to severe, such as mobility and vision problems, fatigue and difficulty thinking. MS is usually diagnosed between the ages of 20-50, but late onset MS can occur in patients over 50 years old. While there is no cure for MS, there are effective treatments that can help reduce the burden of patients’ symptoms and create a positive quality of life. At Renown Health, we have joined the fight against MS through a partnership between advanced neurology programs and providers and our research office. We are proud to offer newly diagnosed MS patients the opportunity to choose between standard care treatment options or participating in an open label clinical trial, the AGNOS study. This study is assessing the impact of a new medication, ofatumumab, as the first disease modifying therapy participants receive for managing relapsing remitting MS, the most common form of MS.
Read More About Multiple Sclerosis Research Opportunities in Northern Nevada