Buscar
Results for 'clinic'
Clear-
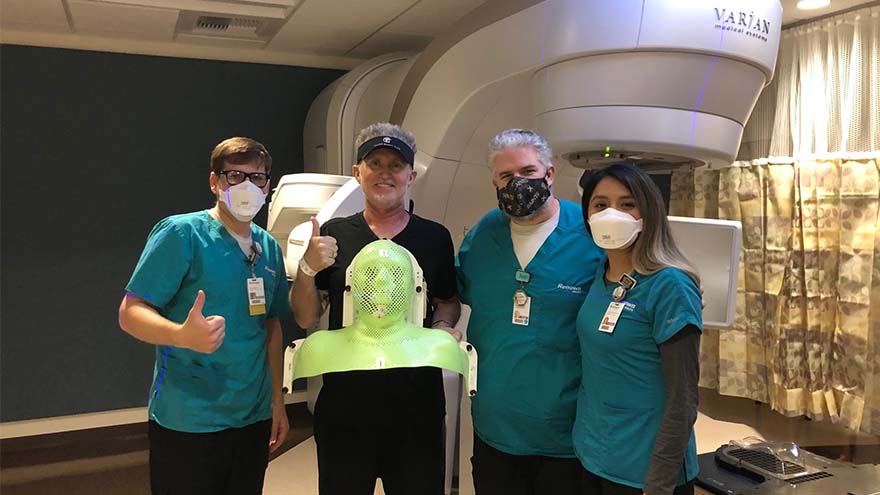
Un diagnóstico de cáncer y una mudanza a Reno
Michael Millman was all set to move to Reno from the Bay Area when he noticed a pimple-like growth on his forehead, and he decided to get biopsied "just in case." It was July 2020, less than six months into the COVID-19 pandemic, when Michael got the call that the biopsy came back cancerous. He was in shock. Still living in the Bay Area at the time, he immediately scheduled to have the basal cell carcinoma removed in August. After the removal, he thought he was in the clear, but a few months later, Michael noticed that his lymph nodes felt weird, and he even cut himself shaving because of some persistent swelling in the area. Given his recent history of skin cancer, Michael immediately scheduled an appointment with a specialist in the Bay Area. "I met with an ear, nose and throat doctor who suggested a fine needle biopsy of my lymph nodes, tongue and an MRI, both with and without contrast," Michael said. "I remember feeling dreadful and that I couldn't believe this was happening yet again." A Hard Decision Michael's squamous cell carcinoma, determined by the pathology report to be significantly influenced by the HPV virus, had metastasized to his lymph nodes on both sides of his neck, and his doctor said it could be stage four cancer. He remembers feeling like he was in quicksand, unsure if he should follow through with his move to Reno, or stay in the Bay Area for treatment. By now, it was early December 2020, and hospitals in the Bay Area and across the world were at limited capacity due to COVID-19. But, in what Michael describes as a positive twist of fate, the San Francisco ear, nose and throat provider he had seen about his biopsy results mentioned that he knew many providers in the oncology department at Renown, including Abhinand Peddada, MD. The San Francisco provider called Dr. Peddada's office with a referral, and Michael even remembers that Renown called him to hear more about his diagnosis before he even got the chance to call them "To be honest, I was feeling shut out in the Bay Area, and Dr. Peddada said he could help me expedite the treatment process," Michael said. "I finally felt a sense of relief." And so began Michael's 7-week chemoradiation cancer treatment program at Renown.
-
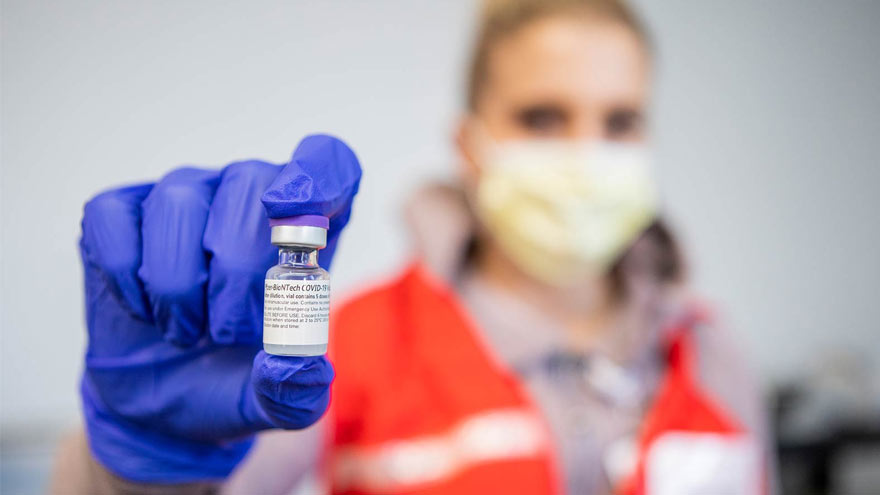
Asesoramiento experto en vacunas contra el COVID-19
With front-line workers receiving the first COVID-19 vaccinations, many of us are feeling hope, but also worry. As a result, we are joining with the Ad Council, the COVID Collaborative, HHS, CDC and NIAID (along with top health and medical organizations) to address your vaccine concerns and questions. Will the vaccine be available to everyone in Nevada? The Nevada Department of Health and Human Services (DHHS) is collaborating with health systems about the use of initially available, limited supplies of COVID-19 vaccines. They will provide guidance on the prioritization order of who will receive the vaccine. This will be based on available quantities, high-risk locations of work and certain other risk factors, and recommendations and guidance for public health agencies. The CDC has provided guidance to initially focus on the following groups: Healthcare personnel likely to be exposed to or treat people with COVID-19, nursing home residents and others in institutional settings; People at risk for severe illness from COVID-19 due to underlying medical conditions; People 65 years of age and older; Other essential workers. I worry the vaccine has been rushed The U.S. national vaccine safety system ensures that all vaccines are as safe as possible, and because vaccines are given to millions of healthy people to prevent serious diseases, they’re held to very high safety standards. COVID-19 vaccines are undergoing a rigorous development process that includes vaccinating tens of thousands of people who participate in a study to generate the needed clinical data. These clinical trials generate scientific data for the FDA to determine the safety and efficacy of each vaccine. It’s worth noting that the clinical studies to establish the safety and efficacy of the Covid-19 vaccines were as big and thorough as recent studies for other licensed vaccines (for example, the shingles vaccine). I'm concerned about the vaccine's side effects The most common side effects are very similar to those seen with most vaccines, such as: sore arms, fevers, and tiredness within 72 hours after the vaccine. These side effects usually mean that the vaccine is generating an immune response, indicating it is working. Short-term side effects observed in the leading COVID-19 vaccine trials include: Injection site pain and redness Fatigue Muscle aches and pains Joint pain Headache I’m afraid I’ll get COVID-19 from the vaccine None of the authorized and recommended COVID-19 vaccines, or COVID-19 vaccines currently in development in the United States, contain the live virus that causes COVID-19. This means that a COVID-19 vaccine cannot make you sick with COVID-19. Can children receive the COVID-19 vaccine? Not at the moment. In early clinical trials for various COVID-19 vaccines, only non-pregnant adults at least 18 years of age participated. However, clinical trials continue to expand those recruited to participate. The groups recommended to receive the vaccines could change in the future. As of now, it is recommended that children do not receive the vaccine. More information will be available from the vaccine manufacturers. I do not believe vaccines are effective Both this disease and the vaccine are new. We don’t know how long protection lasts for those who get infected or those who are vaccinated. What we do know is that COVID-19 has caused very serious illness and death for a lot of people. If you get COVID-19, you also risk giving it to loved ones who may get very sick. Getting a COVID-19 vaccine is a safer choice. The FDA is responsible for making sure that, just like any other medications, any FDA-authorized or approved COVID-19 vaccines are safe and they work. The EUA (Emergency Use Authorization) will not be provided if the FDA feels that the vaccine is unsafe. I can't get vaccines to due to a medical condition Adults of any age with certain underlying medical conditions are at increased risk for severe illness from the virus that causes COVID-19. mRNA COVID-19 vaccines may be administered to people with underlying medical conditions provided they have not had a severe allergic reaction to any of the ingredients in the vaccine. The following information aims to help people in the groups listed below make an informed decision about receiving the mRNA COVID-19 vaccine. It is extremely important to speak with your doctor regarding your specific medical condition, and always follow their strict advice regarding the COVID-19 vaccine, or any other vaccines. Sources: Renown COVID-19 Ad Council COVID Collaborative U.S. Department of Health & Human Services Centers for Disease Control and Prevention National Institute of Allergy and Infectious Disease
-
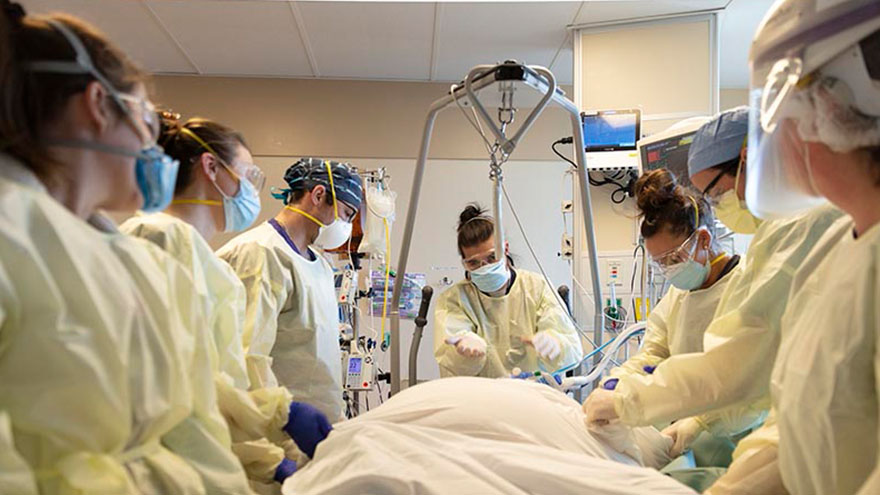
Dos años que no olvidaremos: la COVID-19 en Renown Health
On March 19, 2020, Renown Regional admitted the first patient in need of care while sick with COVID-19. Our providers navigated two years of a pandemic and overcame many challenges while providing the best care for our patients and the community. Anicia Beckwith’s series “The Art of Healing” captured Renown Health during this time. Let's take a look back on the last two years. February 2020: Standing Up the Hospital Incident Command System (HICS) On February 25, 2020, leaders at Renown Health stood up Renown’s Hospital Incident Command System (HICS), a standardized system used to organize response personnel and resources and manage response operations during emergencies and crises. March 2020: Temporary Deployable Medical Structure Placed Outside Renown Regional Emergency Department On March 12, 2020, Renown set up a deployable medical facility to serve as a respiratory illness screening center for emergency room patients at Renown Regional. A similar tent was also set up outside the emergency room at South Meadows Medical Center. This proactive measure helped our teams care for community members with respiratory illness symptoms while protecting patients and staff in the emergency department and other areas of the hospital. Check out photos of the tent here. Read the Reno Gazette Journal Article about the tent here. April 2020: Alternate Care Site at Mill Street Parking Structure at Renown Regional Renown’s HICS team decided to create an Alternate Care Site (ACS) in the Renown Regional Medical Center Mill Street parking structure. The ACS served additional hospitalized patients and allowed caregivers to remain on campus and still have access to existing hospital infrastructure such as lab, pharmacy, imaging, food services and other critical services. After just 10 days of construction, the ACS was completed on April 3, 2020 with space to hold up to 1,400 patients. Check out photos of the ACS under construction here. On Nov. 12, 2020, Renown opened the ACS to serve additional hospitalized patients diagnosed with COVID-19 who were clinically stable or improving. Healthcare workers at Renown cared for hundreds of patients at this site. In early Jan. 2021, the remaining patients returned home. Check out the video of Connie, a patient who received care in the ACS. April and July 2020: The LOVE Sculpture Placed at Renown Regional On April 16, 2020, during a time of darkness and uncertainty, Artown loaned Renown the LOVE sculpture, a one-ton aluminum piece of art created by artist Laura Kimpton and fabricated by Jeff Schomberg. The structure, which originally debuted at Burning Man, was lit up Renown Regional's main entrance on Mill St. Watch a video about the LOVE sculpture’s debut at Renown Regional. On July 13, 2020, thanks to the support of former board chair and community supporter Blake Smith and the Keyser Foundation, the LOVE sculpture is now a permanent fixture at Renown Health. Throughout the pandemic, it has served as a source of inspiration, hope and positivity for our community and care providers. Check out a video of the LOVE is Here to Stay celebration. June 2020: Renown Offers In-House COVID Testing In June 2020, the Renown laboratory team sprang into action to help meet the growing demand for COVID-19 testing amongst Washoe County residents and businesses. Renown invested in expanded staffing and in-house testing capabilities that ensured our teams could swab and process up to 1,000 COVID-19 tests for patients each day. All with results returning within hours. November 2020: Renown Introduces “Hospital At Home” Remote Monitoring In November 2020, six patients at Renown Regional Medical Center and Renown South Meadows Medical Center diagnosed with COVID-19 were outfitted with a remote Hospital at Home monitoring system. Renown clinicians plan to continue using this system to monitor upwards of 1,000 hospitalized patients and lower acuity patients from their homes. December 2020: Renown Administers COVID-19 Vaccines to Health Care Employees On Dec. 17, 2020, Renown began to vaccinate its healthcare workers. Among those receiving the first vaccines was Luis Martinez, a technician on Renown’s Clinical Decision Unit who cared for patients recovering from COVID-19 in the Alternate Care Site field hospital. Read the Reno Gazette Journal article about the COVID-19 vaccine rollout at Renown. January 2021: Renown Administers COVID-19 Vaccines to Community After several weeks of successful employee and volunteer drive-thru vaccination events, Renown supported the Washoe County Health District and the state in vaccinating Washoe County community members. Click here for a playlist of videos featuring Renown Health employees and patients talking about the importance of the COVID-19 vaccine. February 2021: Local Widow Inspires Renown to Change Visitor Supporter Policy Darlene Randolph’s husband Dave spent 17 days hospitalized at Renown Regional Medical Center before losing his battle with COVID-19 on December 13, 2020. Darlene wrote a passionate letter to Renown Health asking for the visitor policy that allowed patients with COVID-19 to receive visitors. In February 2021, Renown hospitals were among the first in the country to lift visitor restrictions for patients with COVID-19 to encourage families to be at the patient's bedside. Read Darlene’s full story here. May 2021: Renown Celebrates Volunteers, Partners and Community Who Aided in Vaccine Efforts In May 2021, Renown administered the last dose of COVID-19 vaccines to community members in Renown’s drive-thru clinic. Between January and May 2021, over 80,000 doses were administered at the drive-thru. View drone footage of this effort here. Click here to see pictures of vaccine volunteers and employees. November 2021: Renown Offers Vaccine for Children Ages 5+ In November 2021, Renown vaccinated children in the Reno-Sparks community with the 2-dose series in a limited round of weekend clinics. The vaccine clinics featured therapy dogs, local mascots and donuts donated by Doughboy’s Donuts. Click here to see pictures of the children’s vaccine clinics and watch a video about the clinics here.
Read More About Two Years We Won't Forget: COVID-19 at Renown Health
-
The Healthy Nevada Project Changed My Life: la historia de una mamá local
Read about Jordan Stiteler, a local mom who says the Healthy Nevada Project provided insights into her family’s genetic makeup — and the likely cause of her dad and great grandfather’s deaths. Now she is changing her life due to her new diagnosis of familial hypercholesterolemia, which will allow her to take steps toward preventing the same fate. Jordan Stiteler’s dad died suddenly of a stroke nearly ten years ago — at only 45 years old. His grandfather died at age 40. Now through the Healthy Nevada Project’s no-cost genetic testing, she is closer to understanding why that may have happened. And she can take proactive steps to prevent the unhealthy symptoms that often lead to a stroke and heart problems. After getting her Geno 2.0 by National Geographic ancestry report, Stiteler got a call from Renown Institute for Heart & Vascular Health Cardiologist and Renown IHI Director of Research, Dr. Christopher Rowan. “They told me that I have FH, which is familial hypercholesterolemia,” she said. “I have genetically very high cholesterol because I have a non-functioning gene that doesn’t get rid of my cholesterol like a normal body would.” Familial Hypercholesterolemia: Simple Life Changes Dr. Rowan told Stiteler it is curable with medication and a change in lifestyle. Stiteler has embraced healthy lifestyle changes by exercising more and eating healthier. “It is so important. Being a mom, I think you have so much more to live for. Having this information has changed my life.” Stiteler feels confident FH affected her Dad. “It is helping my family realize that we need to get tested,” she said. “There were big milestones that my Dad missed. He didn’t get to see either of us get married or have our children. That was huge.” She has become passionate about sharing the need to join the Healthy Nevada Project as her way of helping to prevent other families from possibly going through what she and her family did with the early loss of her Dad. In addition to FH results, the Healthy Nevada Project is returning clinical results on BRCA 1/2 (hereditary breast and ovarian cancer) and Lynch syndrome (colorectal and endometrial cancer) to consenting study participants. To sign up for the Healthy Nevada Project, go to HealthyNV.org. Join the Healthy Nevada Project Recruitment for phase two is still open. In addition to opting in to receive clinical results, participants receive National Geographic’s Geno 2.0 ancestry app at no cost. They also have the chance to pick an additional app for health and wellness after completing a follow-up survey. Learn More or Sign Up
Read More About The Healthy Nevada Project Changed My Life: A Local Mom's Story
-
Farmacéuticos responden preguntas sobre las vacunas contra la COVID-19
Vaccines that provide protection against the COVID-19 virus are bringing us closer to the end of this deadly pandemic. Two different COVID-19 vaccines are currently available in the U.S. today: one from Pfizer and the other from Moderna. Kate Ward, PharmD, BCPS, Director of Clinical Pharmacy at Renown Health and Adam Porath, PharmD, Vice President of Pharmacy at Renown, share what you need to know about these vaccines. When two COVID-19 vaccines were approved by the U.S. Food & Drug Administration (FDA) in December 2020, it was cause for celebration. Why? Because according to the CDC, the vaccines are 94 percent or more effective in providing protection against the COVID-19 virus! Many people are seeking information about the new Moderna and Pfizer vaccines. Below, our pharmacy leaders provide answers to some commonly asked questions. How do the COVID-19 Vaccines Work? The Pfizer and Moderna vaccines are both mRNA vaccines that help your immune system develop antibodies against the COVID-19 virus. The vaccines use messenger RNA, or mRNA, to show our bodies’ protein-making cells how to make the spike proteins of the COVID-19 virus. Our immune system reacts to these spike proteins by creating antibodies that can recognize and destroy them. So when a person is exposed to the virus in the future, they will be less likely to get sick. What are the Differences between the Pfizer and Moderna Vaccines? The Pfizer and Moderna COVID-19 vaccines are very similar, with just a few small differences worth noting. The main difference between the two vaccines is when you should receive your follow-up dose. Patients who receive a first dose of Pfizer should receive their second dose about three weeks later. Those who receive a first dose of Moderna should receive their follow-up vaccination roughly four weeks after their first dose. People 18 years and older can receive the Moderna vaccine while people 16 years and older can receive the Pfizer vaccine. Dosage for the Moderna vaccine is 0.5 ml (100 mcg). Dosage for the Pfizer vaccine is 0.3 ml (30 mcg).
Read More About Pharmacists Answer Questions about the COVID-19 Vaccines
-
Seeking Donors Who Have Recovered from COVID-19 to Donate Plasma
New study, led by physician researchers from Renown Health and University of Nevada, Reno School of Medicine seeks to understand how the immune system responds to COVID-19 with goal of developing a new treatment. Individuals who have recovered from COVID-19 may now be able to help patients currently fighting the infection by donating their plasma. Those who have recovered from the infection may have COVID-19 antibodies in their blood. These antibodies provided one way for their immune systems to fight the virus when they were sick, so their blood may be used to help others fight off the disease through convalescent plasma. Convalescent plasma is a component of blood from recovered patients that may contain precious COVID-19 antibodies. Antibodies are proteins that might help fight the infection. In this study, we will be collecting plasma from patients who have recovered from COVID-19 and investigating its efficacy in helping treat other patients with COVID-19. Convalescent plasma is being investigated for treatment of COVID-19 because there is no approved treatment for the disease and there is information that suggests it might help some patients recover from COVID-19. Renown Health and the University of Nevada, Reno School of Medicine (UNR Med) are leading a study locally to better understand how the body’s immune system responds to the virus, how it presents in northern Nevada and ultimately, aid in developing a new treatment for COVID-19. “Renown and UNR Med are at the forefront of conducting essential research to increase the health and safety of our community,” said Sara Healy, MD, MPH, principal investigator of the study and a pediatric infectious disease physician at Renown Children’s Hospital and UNR Med. “So little is known about effectively treating COVID-19 and we are venturing into new territory. This important study is instrumental in helping us understand the immune systems of people who were affected by COVID-19, and with their help, getting us one step closer to finding a treatment for the disease that has significantly impacted our nation and our community.” “COVID-19 survivors are in a unique and exciting position to be a part of something much bigger than the virus,” said Mark Riddle, MD, DrPH, FISTM, associate investigator of the study and Associate Dean for Clinical Research at UNR Med. “As a participant of this study, not only are you helping us to better understand the disease and the chronic health affects it has long term, but it’s a way to help those suffering from the disease to fight it and hopefully recover. We encourage participation in this important study and invaluable contributions to advancing medicine and our knowledge of COVID-19.” This community-wide study led by physician researchers from Renown Health and UNR Med is a collaborative effort with Vitalant, county and state health districts, Saint Mary’s Medical Center, Northern Nevada Medical Center, Carson Tahoe Health and the VA Sierra Nevada Health Care System, along with the many care providers in our area. Se anima a las personas de entre 18 y 60 años que gocen de buena salud y que se hayan recuperado completamente de la COVID-19 hace dos semanas, como mínimo, a que consideren la posibilidad de donar plasma de convaleciente como parte de este estudio. Hay 332 personas, solo en el condado de Washoe (al 5/1/20) que se han recuperado de la COVID-19 y tienen sistemas inmunitarios que ahora pueden producir anticuerpos para protegerlos de volver a infectarse con el coronavirus. Donated plasma is needed right now, for this clinical trial to determine definitively if this treatment works. Participating in this research study will also make it easier to donate plasma to the Mayo Clinic convalescent plasma program that Renown is a part of in hopes to find a treatment for COVID-19. There is no cost to participate in this study and participation is voluntary. An individual’s decision to participate will not affect their current or future relations with their health care provider(s), health district, or the community. Those who decide to participate are free to withdraw at any time. Los pacientes confirmados de COVID-19 que se hayan recuperado del virus y estén interesados en participar en el estudio deben comunicarse con los coordinadores del proyecto; para ello, pueden llamar a la Oficina de Investigación de Renown al (775) 982-3646 o enviar un correo electrónico a covidplasmascreening@renown.org, de lunes a viernes, de 7:30 a.m. a 5 p.m.. About Renown Health Renown Health is a locally governed and locally owned, not-for-profit integrated healthcare network serving northern Nevada, Lake Tahoe and northeast California. Renown es uno de los mayores empleadores privados de la región y cuenta con una fuerza laboral de más de 7,000. It comprises three acute care hospitals, a rehabilitation hospital, the area’s most comprehensive medical group and urgent care network, and the region’s largest and only locally owned not-for-profit insurance company, Hometown Health. Renown has a long tradition and commitment to continually improve the care and the health of our community. Visite renown.org para obtener más información. About the University of Nevada, Reno School of Medicine The University of Nevada, Reno School of Medicine (UNR Med), Nevada’s first medical school, is a community-based, research-intensive medical school with a statewide vision for a healthy Nevada. Fundada en 1969, la UNR Med está mejorando la salud y el bienestar de todos los residentes de Nevada y sus comunidades mediante excelencia en la educación estudiantil, capacitación de posgrado y atención clínica, investigación con impacto local, nacional y global y una cultura de diversidad e inclusión. For more information, visit med.unr.edu.
Leer más About Seeking Donors Who Have Recovered from COVID-19 to Donate Plasma
-
A Fighting Chance at 24 Weeks Sloans Story
Most babies weigh just one pound and are roughly the size of an eggplant when they reach 24 weeks of development inside the womb. It is a crucial stage when internal organs begin functioning, and the babies' respiratory and central nervous systems are still developing. So, in November 2021 when Kallie Johnson experienced a premature rupture of amniotic fluid around this point in her pregnancy, her care team in Winnemucca decided to transport her via Care Flight to Renown Regional Medical Center. The team at Renown Children’s Hospital immediately began discussing the risks of delivering at 24 weeks with the Johnson family. Moving Forward with Hope Knowing the stakes, Kallie remembers never feeling rushed to decide about delivering her baby preterm. “I felt educated and supported by my care team at Renown throughout our entire stay, starting with the education they provided about what it meant to deliver my baby early,” Kallie said. “The team really helped me make the best decision for myself and my family.” Together, Renown employees and the Johnson family moved forward with a healthy set of nerves and a powerful feeling of hope. Weighing in at one pound 11 ounces, Sloan entered the world on Nov. 19, 2021, via emergency Cesarean section. Her birth was classified as a micro preemie because she was born before week 26 of pregnancy and so small that she fit inside the palm of her father Sterling’s hand. A full-term pregnancy is classified as reaching 39 weeks. A Fighting Chance Called a fighter by many Renown Children’s Hospital care team members, Sloan spent over five months in the neonatal intensive care unit (NICU). She was placed on a ventilator, fed through a feeding tube and monitored 24/7, overcoming daily challenges with the Renown team and her family. As a result of being born prematurely, Sloan developed a grade one brain bleed and a congenital heart defect called patent ductus arteriosus, a persistent opening between two major blood vessels, causing too much blood to flow to the lungs and heart. To meet the oxygen needs of her tiny lungs, Sloan was intubated and developed a severe oral aversion and high-arched palate as a result. The effects would lead to difficult developmental and physical challenges that she still conquers today. Yet, with the help of her care team – including physical, occupational and speech therapists, dieticians and doctors – Sloan continues to make progress every day.