Buscar
-
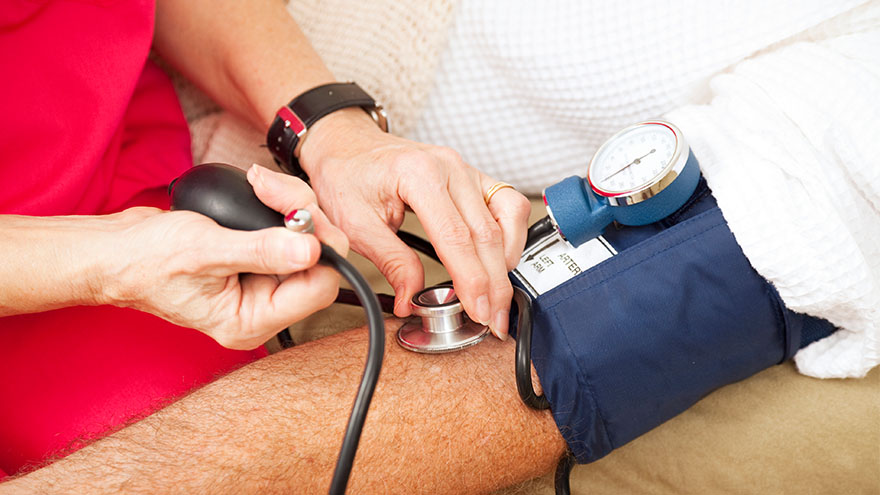
Managing Your Uncontrolled High Blood Pressure
Renown Health, the region's leading cardiology care provider, is offering a clinical trial for eligible patients struggling to control their high blood pressure. Nearly half of adults (119.9 million) in the United States have hypertension, or blood pressure that is higher than normal. Hypertension can put you at risk of other life-threatening disease, such as a heart attack or stroke. There are methods that cardiologists use to manage high blood pressure, but only 1 in 4 adults with hypertension (27.0 million) have their blood pressure under control.* Some patients with high blood pressure experience resistant hypertension, which does not respond well to multiple antihypertensive medications given at the same time. This means that there are many important opportunities for healthcare professionals to explore new ways to treat hypertension. At Renown Health, we lead the region in cardiology care with our technological expertise and patient-centered approach. That is why our cardiology team is partnering with the Renown Research Office to offer the RADIANCE Continued Access Protocol (RADIENCE CAP) clinical trial to eligible patients. RADIANCE CAP is a non-randomized study designed to allow for continued access to ultrasound renal denervation therapy via the Paradise System, and to allow for the on-going collection of safety and effectiveness data in patients with uncontrolled hypertension despite the prescription of antihypertensive medications. The body’s complex communication system between the brain, heart and kidneys can sometimes become overactive, increasing your blood pressure through messages in the nervous system. Renal denervation is a minimally-invasive procedure which reduces activity from the nerves in your kidneys to lower blood pressure. This is the third in a series of renal denervation clinical trials Renown Health has offered to patients with resistant hypertension over the last several years, with over 40 local participants. “All the participants that I have had the pleasure to work with on these studies are very excited and grateful to have this option for helping control their blood pressure” states Lisa English, Lead Clinical Research Coordinator for Cardiology studies at Renown Health. “I love getting to know each one of them and helping on their healthcare journey. We have an amazing team of providers and staff at Renown that go out of their way to make patients experiences positive and the studies successful.” Dr. Michael Bloch, Cardiologist and Principal Investigator for the RADIANCE CAP study at Renown Health’s Institute for Heart and Vascular Health adds, “Despite lifestyle modifications like diet and exercise and the widespread availability of effective and well-tolerated medications, approximately 50% of all people with hypertension have inadequate blood pressure control putting them at risk for stroke, heart failure and kidney disease. As a one-time durable procedure, renal denervation with the Paradise endovascular system from ReCor Medical, Inc. may help millions of patients improve their blood pressure control without necessarily needing to increase their medications.” Our teams of expert providers and researchers are here to support you on your healthcare journey. Talk to your provider about the RADIANCE CAP clinical trial at your next appointment to see if participation may be right for you.
Read More About Managing Your Uncontrolled High Blood Pressure
-
Top 5 Misconceptions About Clinical Trials
There are many misconceptions about clinical research, so we have unpacked a few common myths we hear to help you make an informed decision in your healthcare. Misconception #1: If I join a clinical trial, I’ll just be a guinea pig. Quite the opposite is true! Through honest and respectful conversation, we ensure all participants are informed of the benefits and risks associated with the clinical trial during the informed consent process. Being in a clinical trial is voluntary, and we respect our patients’ decision to join or decline to participate in the clinical trial. You can always change your mind at any time as well. When patients join a clinical trial, they receive an additional team of healthcare professionals, including additional physicians, pharmacists and research coordinators, dedicated to their safety and well-being. This means that clinical trial participants often receive more support than they would in the standard treatment setting. Misconception #2: Clinical trials are too dangerous because they use new treatments that haven’t been tested. We recognize that there are different levels of risk associated with participating in a clinical trial depending on the type of study. However, new treatments are only reviewed through clinical trials after they have gone through extensive testing. New treatments that do not show promising results for safety and potential benefit during laboratory testing do not receive approval to begin clinical trials. Your research team reviews any expected benefits and risks identified from previous studies during the informed consent process, as well as any updates that occur throughout the duration of the clinical trial. The research team stays in close contact with you during the entire process, documenting and treating any side effects that you experience for both your safety and the safety of participants like you. Misconception #3: I don't want to join a trial because I could be wasting my time receiving a placebo. A placebo is a substance that has no therapeutic effect, sometimes called a “sugar pill.” Participants who receive a placebo during a clinical trial are very important, helping researchers definitively determine the specific good and bad effects of the new medication. Many clinical trials that involve a placebo also offer what is called an open label extension or cross-over study. Cross-over studies ensure that anyone taking the placebo can begin receiving the new medication, often for several years. Cross-over studies help clinician researchers understand the long-term effects of a medication while also giving patients free access to novel care for several months and even years.
-
Monkeypox: A Renown Expert Weighs In
Renown Health is closely following the national outbreak of the monkeypox virus and urging healthcare providers to be alert for patients with illnesses associated with a rash. In working with the Washoe County Health District (WCHD), Renown is closely monitoring the spread of monkeypox in the community and looking to prevent and reduce the spread of monkeypox. To help to ease worries, we consulted with Paul De Leon, Infection Preventionist at Renown Health. What Exactly is Monkeypox? Monkeypox is a rare viral illness caused by the monkeypox virus — the same family of viruses that causes Smallpox. Although symptoms are similar to Smallpox, monkeypox symptoms are milder and rarely fatal. However, it's important to mention that this virus can be more severe for these susceptible groups: Immunocompromised Pregnant women A fetus or newborn baby Women who are breastfeeding Young children Those with severe skin diseases such as eczema How is Monkeypox Transmitted? The monkeypox virus is not easily transmitted but occurs through sustained person to person close contact with an infected individual. Monkeypox can also be transmitted through direct contact with infectious rash, scabs, or body fluids. Monkeypox can also be spread through prolonged intimate physical contact, such as kissing, cuddling or sex. Lastly, monkeypox can be spread through contaminated linens or bedding. Transmission through respiratory secretions is uncommon but has been reported after prolonged face-to-face contact with symptomatic individuals. In addition, pregnant women can spread the virus to their fetuses through the placenta. Monkeypox Testing If you think you have monkeypox, contact your primary care physician or other medical providers to obtain testing. Notify the provider ahead of time before entering the physical office. Signs & Symptoms This current outbreak of West African monkeypox does not have the typical presentation of classic monkeypox. Symptoms usually appear one to three weeks after infection and include: Pimple-like rash or blisters on the face, inside the mouth, and on other areas of the body, like the hands, feet, chest, genitals, or anus. The rash will go through serval stages, including scabs, before healing and may be painful or itchy. Other symptoms of monkeypox can include: Fever Headache Muscle aches and backache Swollen lymph nodes Chills Exhaustion Respiratory symptoms such as sore throat, nasal congestion, or cough Symptoms of monkeypox may occur before or after a rash with some individuals only report experience a rash. Individuals with monkeypox are infectious once symptoms begin and remain infectious until lesions form scabs, scabs fall off, and a fresh layer of skin forms. The illness typically lasts 2-4 weeks.
-
¿Qué significa participar en un estudio clínico?
Participating in a clinical trial is voluntary and a personal choice. Clinical trials are research studies that involve people and are an important part of patient care. What is a clinical trial? Clinical trials are research studies that involve people, and they are an important part of patient care. There are several different types of clinical trials; some are designed to understand trends in a disease or identify better ways to diagnose a condition, while others determine if a new treatment is safe and works when treating, improving or preventing a health condition. There are over 400,000 clinical trials currently being conducted in the United States, and even more across the world. This includes health conditions such as heart failure, cancer, Parkinson’s Disease, respiratory conditions like COPD, common infections, cystic fibrosis, and many more. Clinical trials lead the healthcare industry to new discoveries that contribute to reliable and exact care, improving healthcare quality and saving lives. Clinical trials are conducted by a team of researchers, including doctors, pharmacists and clinical research coordinators. These research teams are highly skilled in their specialty areas, often providing traditional patient care and seeing research patients in the same day. These teams are responsible for making sure the clinical trial is completed correctly, and their patients are their top priority. Why should I consider participating in a clinical trial? Participating in a clinical trial is voluntary and a personal choice. There are many reasons why patients decide to get involved in clinical research. While many clinical trials are designed for patients who have a certain health condition, many studies also ask healthy volunteers to contribute in order to compare health outcomes. Clinical trials are also for patients at all different stages of their diagnosis. Depending on the specific study, the patient may receive access to a new cutting-edge treatment before it is widely available. When patients join a clinical trial, the research team becomes a health partner dedicated to their health and well-being. When patients join a clinical trial, they make an informed decision in their healthcare by weighing all available options in addition to routine treatments. Research participants know that they are contributing meaningfully and helping other patients like them. Where can I find more information about clinical trials at Renown Health? Renown Health’s mission is to make a genuine difference in the health and well-being of the communities we serve. Renown’s clinical trial portfolio offers leading care options to patients in northern Nevada, close to home, in a variety of specialties. Contact the Renown Clinical Research Office for more information on clinical trials available to you!
Read More About What Does It Mean to Participate in a Clinical Trial?
-
What Every Woman Needs to Know About Dense Breast Tissue
In honor of International Women’s Day, we’re working to spread the word about taking care of your breast health and encouraging the women in your life to do the same. Heather Reimer is on a mission — a mission to educate women everywhere about breast tissue type. For women with dense breasts, knowing your breast tissue type is absolutely critical, as cancers embedded in dense breast tissue are not always detectable with a mammogram alone. Dense breast tissue requires a breast ultrasound screening to get a complete breast health picture. Whole Breast Ultrasound for Dense Breast Tissue Heather knows this firsthand. She has dense breasts, and in this video she shares her story about finding breast cancer during a breast ultrasound screening — cancer that went undetected with her mammogram screening alone. As a result of that experience, Heather founded Each One. Tell One. — a movement to encourage women to pass along this information to others and to prompt those with dense breast and implants to consult with their doctor to schedule a whole breast ultrasound screening. To schedule a mammogram or a whole breast ultrasound, call 775-982-8100.
Read More About What Every Woman Needs to Know About Dense Breast Tissue